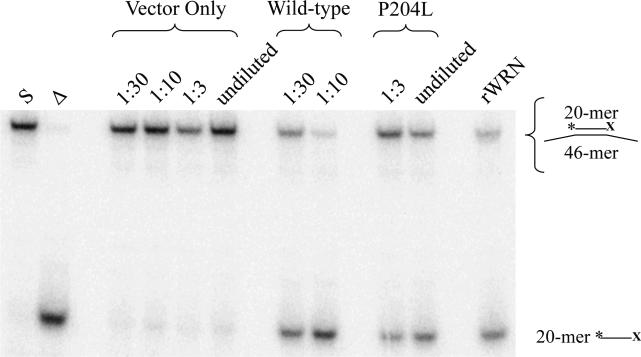
Fig. 6. Helicase activity of P204L WRN in the absence of exonuclease function.
Wild-type and P204L immune precipitates were assayed for helicase activities at the indicated dilutions. Equivalent amounts of WRN protein, as determined by Western blot analysis were assayed. Helicase reactions were performed with a non-degradable substrate in which the 3'end of the *20-mer is blocked (*20X) to prevent the action of WRN exonuclease. Lane 1: (S) *20X/46 substrate; lane 2: (Δ) heat-denatured *20X/46 substrate; lanes 3-6: vector only control; lanes 7-8: wild-type WRN; lanes 9-10: P204L WRN; lane 11: (rWRN) purified recombinant WRN. Helicase activity was determined by comparing the intensity of the lower band, *20X, to the sum of upper and lower band intensities, and found to be reduced 1.6-fold in P204L WRN relative to wild-type.