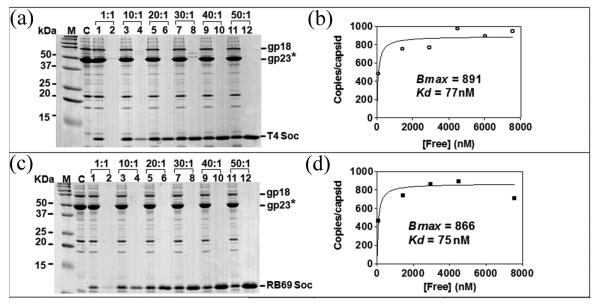
Fig. 3.
Binding of T4 and RB49 Soc proteins to Hoc−Soc− T4 capsids. Binding of Soc proteins to Hoc−Soc− phage and quantitative analyses were performed as described in Materials and Methods. Panels (a) and (c) lane M, molecular mass standards; C, control Hoc−Soc− phage; lanes 1, 3, 5, 7, 9, and 11 show capsid-bound Soc; lanes 2, 4, 6, 8, 10, and 12 show unbound Soc. The positions of gp18 (tail sheath protein), gp23* (major capsid protein), and Soc are labeled. The saturation binding curves (panels (b) and (d)) were constructed and the apparent Kd (association constant) and Bmax (maximum copy number per phage particle) were determined using the GraphPad PRISM-4 software.