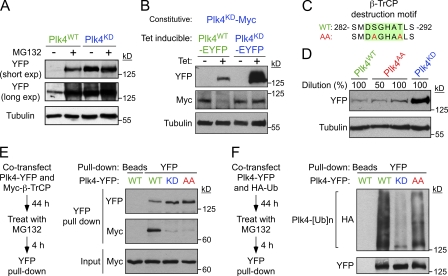
Figure 2.
Phosphorylation of the β-TrCP–binding motif has a minor effect on the stability of mouse Plk4. (A) Cells were treated with or without the proteasome inhibitor MG132 for 8 h and immunoblotted to determine the expression level of Plk4WT and Plk4KD. (B) Expression of Plk4WT-EYFP or Plk4KD-EYFP was induced for 24 h in cells constitutively expressing Plk4KD-Myc. The expression level of the Myc and EYFP-tagged Plk4 transgenes was subsequently determined by immunoblotting. (C) The Plk4AA mutant possesses two mutations that prevent phosphorylation of the β-TrCP–binding motif (highlighted in green). (D) Immunoblot shows a modest increase in the stability of Plk4AA. (E) HEK 293 cells were cotransfected with Plk4-EYFP and Myc–β-TrCP and treated with MG132 for 4 h. Plk4-EYFP was purified using GBP-coupled beads or beads alone, and protein complexes were analyzed by immunoblotting. (F) HEK 293 cells were cotransfected with Plk4-EYFP and HA-ubiquitin and treated with MG132 for 4 h. Plk4-EYFP was purified using GBP-coupled beads or beads alone, and protein complexes were analyzed by immunoblotting.