Abstract
Acute loss of spinal cord vascularity followed by an endogenous adaptive angiogenic response with concomitant microvascular dysfunction is a hallmark of traumatic spinal cord injury (SCI). Recently, the potent vasoactive factor vascular endothelial growth factor (VEGF) has received much attention as a putative therapeutic for the treatment of various neurodegenerative disorders, including SCI. Exogenous VEGF exerts both protective and destabilizing effects on microvascular elements and tissue following SCI but the role of endogenous VEGF is unclear. In the present study, we systemically applied a potent and well characterized soluble VEGF antagonist to adult C57Bl/6 mice post-SCI to elucidate the relative contribution of VEGF on the acute evolving microvascular response and its impact on functional recovery. While the VEGF Trap did not alter vascular density in the injury epicenter or penumbra, an overall increase in the number of Griffonia simplicifolia isolectin-B4 bound microvessels was observed, suggesting a VEGF-dependency to more subtle aspects of endothelial plasticity post-SCI. Neutralizing endogenous VEGF neither attenuated nor exacerbated chronic histopathology or functional recovery. These results support the idea that overall, endogenous VEGF is not neuroprotective or detrimental following traumatic SCI. Furthermore, they suggest that angiogenesis in traumatically injured spinal tissue is regulated by multiple effectors and is not limited by endogenous VEGF activation of affected spinal microvessels.
Keywords: VEGF Trap, Aflibercept, intravital lectin, neovascularization, endothelial, Basso Mouse Scale for Locomotion (BMS)
INTRODUCTION
Traumatic spinal cord injury (SCI) results in both immediate loss of spinal vascular support and the initiation of multiple molecular cascades resulting in continued microvascular plasticity and dysfunction, which persist for days to weeks. The immediate “traumatic phase” of vascular disruption and the subsequent compromise of perfusion contribute to the profound loss of neural elements. This is caused by the high metabolic demand of spinal gray matter and the especially intricate anatomy of gray matter microvascular beds (Ducker and Assenmacher, 1969). Tissue ischemia is caused by physical destruction of microvasculature, and exacerbated by petichial hemorrhage, vasogenic edema (Yang et al., 1994), excitotoxicity (Bullock and Fujisawa, 1992), and loss of penumbral blood flow due to onset of vasospasm/vasoconstriction (Ducker and Assenmacher, 1969). This prolonged ischemia initiates an endogenous pathoangiogenic response lasting for days to weeks post-SCI, giving rise to a neovascularization of affected spinal tissue (Whetstone et al., 2003; Benton et al., 2008a; Beggs and Waggener, 1979; Zhang and Guth, 1997; Imperato-Kalmar et al., 1997). These newly formed vascular networks exhibit multiple pathologic attributes including decreased glucose transport potential, increased blood-spinal cord barrier (BSCB) permeability, abnormal glycocalycal phenotype, and altered integrity of tight-junctions (Noble and Wrathall, 1989; Noble et al., 1996; Whetstone et al., 2003; Popovich et al., 1996; Benton et al., 2008a). A number of vasoactive molecules are thought to participate in this vascular pathophysiology specific to SCI, including the potent vasoactive factor vascular endothelial growth factor (VEGF) (Sharma, 2005). However, the molecular control of these microvascular responses following SCI remains unclear.
Immediately following traumatic SCI, tissue levels of VEGF mRNA (Skold et al., 2000; Bartholdi et al., 1997) and protein (Vaquero et al., 1999; Akiyama et al., 2004) are dramatically upregulated, with levels normalizing by 14 days post-injury (Vaquero et al., 1999). While the temporal profile of this increase in VEGF occurs coincident with vascular plasticity and BSCB pathophysiology (Loy et al., 2002; Casella et al., 2002; Whetstone et al., 2003; Benton et al., 2008a; Bartholdi et al., 1997; Imperato-Kalmar et al., 1997; Beggs and Waggener, 1979), it is unknown to what degree VEGF mediates these vascular events following traumatic SCI. Acute injection of exogenous VEGF following contusive injury in the adult rat can be neuroprotective (Widenfalk et al., 2003) or can exacerbate tissue loss with no enhanced vascular protection (Benton and Whittemore, 2003). More recently, indirect evidence of the deleterious vasoactive actions of VEGF in the context of traumatic SCI has been provided (Akiyama et al., 2004). To date, no report exists regarding the role of endogenous VEGF or the effect of direct antagonism of VEGF in SCI. Thus, the purpose of the current study was to directly antagonize VEGF action in the injured spinal cord using systemic administration of a neutralizing VEGF Trap and examine the subsequent effects on vascular plasticity, tissue sparing and recovery of function following contusive SCI.
MATERIAL AND METHODS
Contusive Mouse SCI
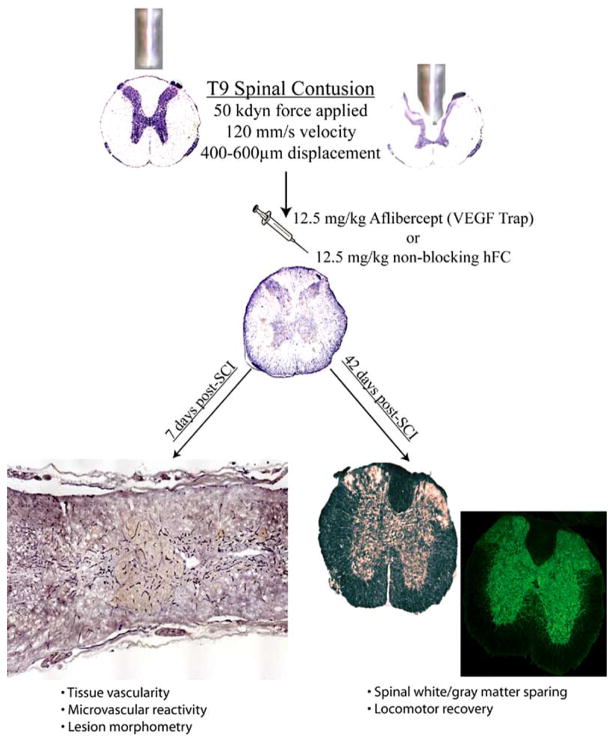
All surgical intervention, perioperative care, and treatment of all animals used in this study were in strict accordance with the PHS Policy on Humane Care and Use of Laboratory Animals and approved by the University of Louisville IACUC committee. Contusive spinal cord injuries (T9/10 spinal segment) of moderate severity (50 kdyn force/500–600 μm displacement) were performed on a total of 26 adult female C57Bl/6 mice (18–20 g, Harlan) as previously described (Benton et al., 2008a).
Administration of VEGF Trap
Injured mice were then randomly divided into two treatment groups receiving a single intravenous bolus of VEGF Trap (Aflibercept, 12.5 mg/kg, n = 13; a generous gift from Regeneron Pharmaceuticals Inc., Tarrytown, NY) or hFc control peptide (12.5 mg/kg, n = 13) at the time of SCI. The VEGF Trap (R1R2) is a fully soluble VEGF decoy created by fusing the immunoglobulin 2 domain of VEGFR1 with the immunoglobulin domain of VEGFR2 (Holash et al., 2002). VEGF Trap also comprises the Fc domain of human IgG1, which induces forced homodimerization of the extracellular VEGF receptor domains. VEGF Trap has a kD of ~pM and superior pharmacokinetic characteristics (t1/2 of ~20 hours in a mouse), ensuring that endogenous VEGF can be neutralized of the first post-injury week with a single loading dose. VEGF Trap exhibits potent anti-tumor effects in vivo by interruption of malignant neovascularization and is currently in clinical trials for this application (Rudge et al., 2005). More recently, systemic administration of VEGF Trap has been shown to decrease detrimental aspects of pathoangiogenesis in retinal vascular beds (Shah et al., 2006; Saishin et al., 2003), suggesting efficacy at the level of the blood-CNS barrier.
Intravital Labeling of Spinal Microvasculature
To assess acute spinal microvascular plasticity, neovascularization and tissue sparing, on day 7 post-SCI, a subset of mice in each group (VEGF Trap/n=5, hFc/n=5) were deeply anesthetized. Next, 100 μg/50 μl of FITC-conjugated Griffonia simplicifolia isolectin B4 (FITC-IB4, L-9381; Sigma, St. Louis, MO) was delivered systemically by intravenous injection via the right external jugular vein and allowed to circulate for 15 minutes. Mice were then transcardially perfused with 10 ml of saline followed by 15 ml of 4% paraformaldehyde (PFA). Spinal cords were dissected and longitudinally sectioned at 20 Nm on a cryostat, slide-mounted, and stored at −80°C until use.
Immunohistochemical analyses and quantitative assessment of microvascular density-To determine vascular density in injury epicenters, vascular endothelial cells (ECs) were identified using a monoclonal rat anti-PECAM-1 antibody (#550274, 1: 50, BD Pharmingen, San Diego, CA). SCI epicenters were identified by quantification of extravascular laminin deposition using polyclonal rabbit anti-laminin (L9393, 1: 100, Sigma, St. Louis, MO)(Benton et al., 2008a; Whetstone et al., 2003). The density of neovascular structure in affected spinal tissue was examined by analyzing the density of luminal binding of FITC-IB4, which identifies activated/angiogenic blood vessels post-SCI (Benton et al., 2008a). Primary antibodies were applied in 0.1M TBS, 0.1% Triton X-100, 0.5% BSA, and 5% normal donkey serum overnight in a humidified chamber at 4°C and epitopes visualized using Rhodamine- (TRITC; 1: 200 dilution) or AMCA- (1: 100 dilution) conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). All photomicrographs demonstrating immunostained spinal cord sections were captured using a Nikon TE 300 inverted microscope equipped with a Spot CCD camera and capture software (Diagnostic Instruments Inc., Sterling Heights, MI). Quantitative assessment of intravascular IB4 binding, vascular EC immunohistochemistry, and lesion area 7 days post-SCI was accomplished as previously described (Benton et al., 2008a) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Briefly, every 5th longitudinal section from each experimental case was processed for stated markers and the epicenter for each case was defined and converted to an area of interest (AOI; Fig. 1) and pixel area of each AOI was automatically calculated by the software. The density of epicenter binding/staining for each image was then calculated by ratio of the total pixels of binding/staining for each measure over the total pixels in the AOI area. Penumbral data were obtained by calculating the density in a 500 μm area bordering each epicenter AOI. All quantitative data for the 7 day post-SCI analyses are expressed as mean ± s.d. and statistical analysis of these data was accomplished using a Student’s t-test.
Fig. 1.
Schema of experimental protocol.
Chronic Assessment of Functional Recovery
The remainder of the mice (n=7/hFc control, n=8/VEGF Trap) were allowed to recover for 6 weeks post-SCI and were assessed weekly for locomotor function using the Basso Mouse Scale (BMS) (Basso et al., 2006). Briefly, locomotor function in spinal cord injured mice was determined during weekly sessions post-SCI by two experienced raters presented with animals in random order. Mice are placed in a round field with a smooth bottom and allowed to freely ambulate for a period of 4 minutes. The low end of the scale (0–4) is characterized by individual joint movements, whereas the intermediate (4–6) and high (6–9) ends of the scale are characterized by weight support and coordination and paw position, respectively. At the end of the experimental period (day 42 post-SCI), mice were euthanized and perfused as described above. A total of 1 cm of spinal cord tissue containing the injury site at its center was dissected, blocked, and sectioned transversely at 20 μm on a cryostat. Spared gray matter was assessed by immunohistochemistry performed as stated above using polyclonal rabbit anti-Map2 (AB5622, 1: 250, Chemicon Inc., Temecula, CA). Every 25th section (≈500 μm resolution) was processed for Map2-immunoreactivity and images captured as stated above. Quantitative per area gray matter preservation was obtained by converting the total spinal cord section to an AOI and calculating the percentage of that area occupied by Map2-immunoreactivity. Spared white matter was assessed eriochrome cyanine staining as described elsewhere (Rabchevsky et al., 2001) in a manner similar to that used to assess gray matter sparing. All quantitative data for the 42 day post-SCI analyses are expressed as mean ± s.d. and statistical analysis of these data was performed by two-way ANOVA with a Tukey HSD post hoc t-test.
RESULTS
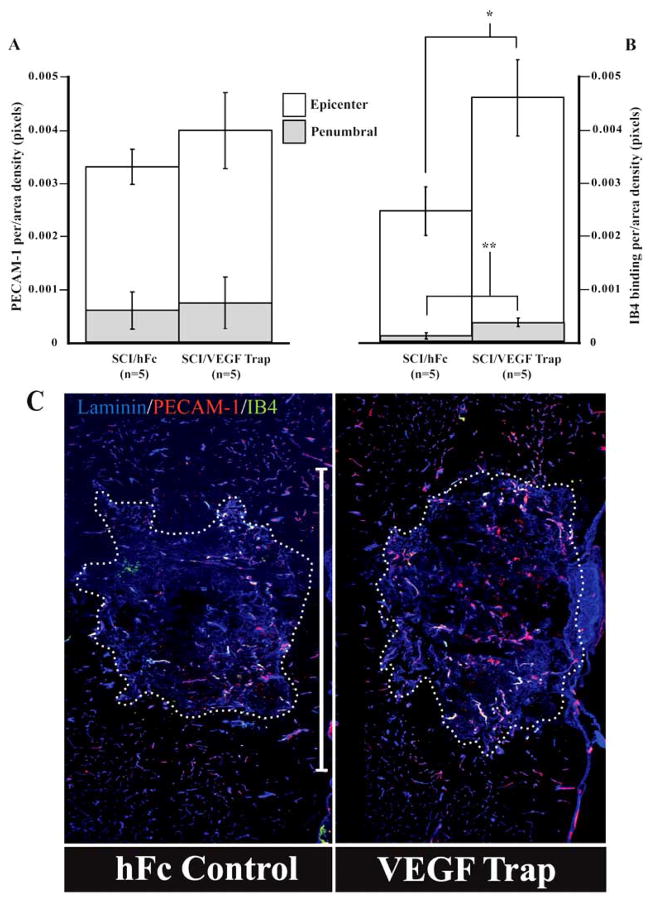
Previous studies have shown that by 7 days post-SCI, epicenter vascularity is significantly greater than that observed 1 day following injury, consistent with an adaptive angiogenesis (Whetstone et al., 2003; Benton et al., 2008a). VEGF Trap treatment did not alter total vascularity as measured by PECAM-1 immunodensity in the injury epicenter or penumbral zones (Fig. 2A). This result suggests that VEGF expressed at the site of SCI is not essential for the SCI-induced neovascular response in and around the injury site. Despite having no effect on total parenchymal vascularity, significantly more of the regenerated blood vessels both within the lesion/heterodomain proper (t = −5.61, df = 7, P < 0.001) and penumbral zones (t = −5.94, df = 7, P < 0.001) expressed luminal affinity for IB4 (Fig. 2B). This result suggests that the VEGF Trap is biologically active in the injured cord and that, surprisingly, endogenous VEGF alters EC plasticity in a pattern that appears to modulate the functional status of neovascular beds in and around the injury site, as luminal IB4 affinity appears to be related to the maturation state of newly formed blood vessels in the injured spinal cord (Benton et al., 2008a).
Fig. 2.
Neutralizing endogenous VEGF increases neovascular profiles 7 days post-SCI. Total vascularity (PECAM-1-immunoreactivity) and IB4 binding was assessed in injury epicenters (C; hatched line) and in penumbral tissue (i.e., a 500μm zone surrounding epicenters). Quantitative data show total vascularity to be unaffected by VEGF-Trap 7 days post-SCI (A). By contrast, vascular activation/angiogenesis as demonstrated by IB4-bound microvessels is significantly increased in the injury epicenter and penumbra (B). All quantitative data are the mean ± s.d. (* P = 0.0008, ** P = 0.0006) Scale bar (C) = 1mm.
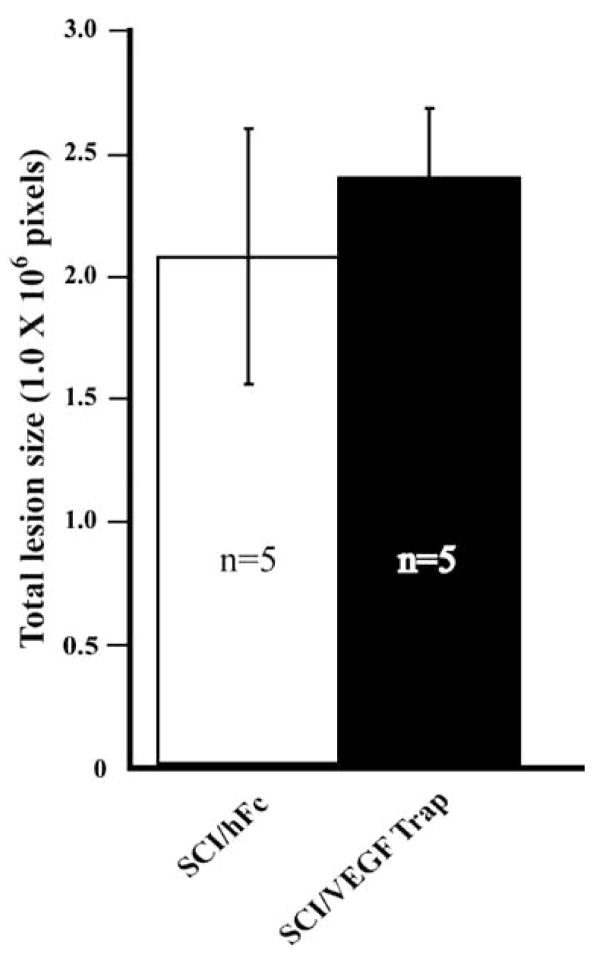
While little is known about how the maturation of angiogenic CNS microvessels is manifested by luminal phenotype, previous findings have suggested that luminal glycocalycal phenotype mediates inflammation contributing to histopathology following SCI (Noble et al., 1996). To address these possibilities, we analyzed lesion sizes as assessed by the pathologic deposition of extravascular laminin, an area for epicenter histopathology referred to as the “heterodomain” (Whetstone et al., 2003) (Fig. 2C; hatched outline). We found that sub-acute (7 day post-SCI) histopathology is not affected by the VEGF Trap treatment or the increased presence of IB4+ neovascular structure (Fig. 3). Furthermore, VEGF Trap treatment did not appear to alter inflammatory responses at this time point as determined by a quantitative assessment of Iba1-immunoreactive activated microglia/macrophages at the injury sites as well as in penumbral zones of the lesion (data not shown).
Fig. 3.
Lesion sizes are unaffected by VEGF Trap treatment at 7 days post-SCI. Areas of pathologic tissue transformation (see Fig. (2); hatched outline) were quantified. Blockade of endogenous VEGF acutely had no chronic effect on lesion evolution. All quantitative data are represented as the mean ± s.d.
Histopathology was also assessed in mice that survived 42 days post-injury. Overall, lesion lengths were found to be appropriate for the C57BL/6 strain at this time point post-SCI (Kigerl et al., 2006) suggesting consistent injury severities for mice in both experimental groups. Spinal gray matter was nearly completely lost at the injury epicenter, with significant histopathology extending approximately 2 mm rostral and caudal to the epicenter (Fig. 4A), with similar observations were made for the expansion of demyelinated white matter (Fig. 4B). Neither white nor gray matter loss post-SCI was significantly affected by treatment with the VEGF Trap. Terminal locomotor scores were statistically identical in each experimental group (Fig. 4C), consistent with histologic data.
Fig. 4.
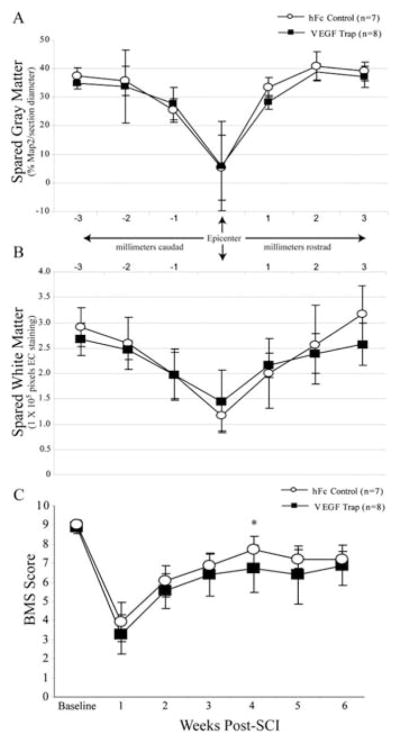
Neutralizing endogenous VEGF does not alter chronic histopathology or alter locomotor recovery following SCI. Spared spinal gray (A) and white (B) matter was analyzed 42 days post-SCI, with no effect observed with VEGF Trap treatment. Similarly, no difference was observed in terminal functional outcome as assessed by BMS evaluation (C). The only exception was a statistically significant decrease in BMS scores in the VEGF Trap treated group at 4 weeks post-SCI (P < 0.05), which was not observed in subsequent evaluation sessions. All data are represented as the mean ± s.d.
DISCUSSION
While somewhat unexpected, the relative insensitivity of the spinal microvascular plexus to VEGF Trap mediated VEGF antagonism is consistent with previous findings. In peripheral tissues, the responsiveness of vessels to VEGF blockade is attenuated with age (Baffert et al., 2004), an effect that was postulated be related to diminished VEGF receptor expression. Recent data show EC mRNA expression of VEGF-R1 (flt-1) and VEGF-R2 (flk-1) decreased by 24 hours post-SCI (Benton et al., 2008b). Capillaries in the intact adult cortex are the least dependent upon VEGF for their survival/maintenance as compared to vascular beds in other organs (Kamba et al., 2006). Despite this, VEGF-R1 does appear to drive pathoangiogenesis in mild traumatic brain injury (TBI) (Krum et al., 2008). Furthermore, in a model of retinal neovascularization, pathologic choroidal vascular plasticity is also VEGF-dependent (Saishin et al., 2003). However, this dependency appears to be quite complex as exemplified by recent findings in stroke. Administration of VEGF blocking antibodies following focal cortical ischemia ameliorates blood-brain barrier (BBB) leakage (Chi et al., 2007). This therapeutic action appears to be VEGF isoform specific, as addition of exogenous VEGF-B actually reduces stroke infarct volume with no change in BBB permeability (Li et al., 2008). Results from the present study would suggest a similarly complex role for VEGF in vascular remodeling following traumatic SCI. Thus, affected spinal microvessels may not exhibit the same requirement for VEGF activity as is observed following TBI or in other pathologically activated CNS vascular beds and/or its requirement is isoform specific.
Despite having no impact on sub-acute vascularity in injury epicenters, VEGF antagonism alters the anatomy and/or physiology of regenerated blood vessels after SCI. It is possible that the increased affinity for IB4 in affected vasculature is merely the result of enhanced perfusion of neovascular elements in the injured spinal cord. Blockade of VEGF in activated tumor vessels using an approach similar to that employed in the current study results in increased perfusion pressures and improved blood flow in the tumor stroma (Tong et al., 2004). This would be consistent with the “normalization” of plastic microvascular plexi by VEGF antagonism, which is an emerging concept in the context of tumor neovascularization (Fukumura and Jain, 2007). Alternatively, VEGF Trap may alter neovascular phenotype in the absence of functional changes. VEGF is known to alter the functional luminal phenotype of extra-CNS microvessels in vivo (Melder et al., 1996) as well as induce disruption of the endothelial glycocalyx (Fu and Shen, 2003). Current results would most directly support this latter explanation.
Fundamentally, present data highlight the complexity of vascular regulation after SCI, a precedent for which exists in peripheral tissue pathology. Specifically, comparable micro-vascular resistance to VEGF-R1 and VEGF-R2 antagonism in pathologic angiogenesis is currently of intense investigation, especially in the context of tumor neovascularization (Shojaei and Ferrara, 2008; Bergers and Hanahan, 2008). To date, several explanations for this phenomenon have been proposed, which fall under the conceptual categories of adaptive/evasive resistance and intrinsic non-responsiveness (Bergers and Hanahan, 2008). Several of these possibilities are quite plausible in the context of SCI. For example, several alternative pro-angiogenic pathways are induced in solid tumors and appear to contribute to the circumvention of an absolute dependence on VEGF for neovascularization. These include fibroblast growth factor (Fgf) (Casanovas et al., 2005), interleukin 8 (IL8) (Mizukami et al., 2005), and platelet derived growth factor-alpha (PDGFA) (Fernando et al., 2008). Importantly, all of these pro-angiogenic cytokines are upregulated in injured/inflamed spinal tissue (Tripathi and McTigue, 2008; Sun et al., 2008; Ishizu et al., 2005). It is possible that a comparable “redirection” of the endogenous angiogenic response may occur in SCI, with increased activation of these angiogenic pathways in affected microvascular ECs.
Unbiased transcriptional screening of ECs isolated from tumor microvasculature has identified a number of novel regulators of tumor neovascularization (St et al., 2000). Of those identified, Delta-like ligand 4 (DLL4) was robustly enriched in tumor ECs, suggesting a role for Notch signaling in vascular activation in solid tumors. Indeed, this pathway appears to be quite relevant to tumor neovascularization refractory to VEGF blockade (for review see Thurston and Kitajewski, 2008). Blockade of DLL4 reduces tumor size by disrupting microvascular function and had additive effects when combined with anti-VEGF therapy (Ridgway et al., 2006; Noguera-Troise et al., 2006). Interestingly, spinal microvascular ECs express detectable levels of DLL4 acutely following SCI (unpublished observations) suggesting a comparable co-stimulatory role for Notch in angiogenesis in the injured spinal cord. Currently, studies are underway to determine to what extent Notch activation regulates EC survival and/or plasticity following SCI.
Finally, in many pathoangiogenic contexts it is likely that lack of complete efficacy of VEGF-R1 and –R2 blockade may be due to incomplete suppression of VEGF signaling in activated ECs. This is most likely due to expression of neuropilin1 (NRP1) receptor, which effects vascular remodeling both independently and in combination with VEGF-R2 (Pan et al., 2007). Furthermore, very recent evidence has identified a novel VEGF receptor isoform (i.e. VEGF-R3), which appears to be critical for the neovascular response in solid tumors (Su et al., 2008; Tammela et al., 2008; Petrova et al., 2008). To date, no data exist regarding EC expression of NRP1 in the injured spinal cord, although it has recently been implicated in regenerative neuronal responses following SCI (Mire et al., 2008).
In summary, the results from the current study demonstrate the complexity of the molecular control of micro-vascular responses to SCI. Further, they suggest targeting a single molecule as a therapeutic intervention might not result in dramatic modulation of pathoangiogenic cascades. As other molecular effectors of pathoangiogenesis in the injured/diseased CNS are identified, future experiments utilizing combinatorial approaches, of which VEGF signaling will likely be a component, may have enhanced potential as viable therapeutic strategies to stabilize and improve microvascular function in CNS pathology.
Acknowledgments
The authors wish to thank Mrs. Christine Nunn and Mrs. Kim Fentress for superb surgical support and perioperative care of experimental animals. Thanks also to Ms. Darlene Burke for assistance with statistical analyses. The authors wish to also recognize Regeneron Pharmaceuticals Inc. for the generous gift of Aflibercept (VEGF Trap) and Fc control and helpful technical advice for its use. Thanks also to Dr. Stanley J. Wiegand for his helpful critique of the current manuscript. This research was supported by NS045734, Norton Healthcare, RR15576, and the Commonwealth of Kentucky Challenge for Excellence. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Akiyama C, Yuguchi T, Nishio M, Tomishima T, Fujinaka T, Taniguchi M, Nakajima Y, Kohmura E, Yoshimine T. Src family kinase inhibitor PP1 reduces secondary damage after spinal cord compression in rats. J Neurotrauma. 2004;21:923–931. doi: 10.1089/0897715041526230. [DOI] [PubMed] [Google Scholar]
- Baffert F, Thurston G, Rochon-Duck M, Le T, Brekken R, McDonald DM. Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94:984–992. doi: 10.1161/01.RES.0000125295.43813.1F. [DOI] [PubMed] [Google Scholar]
- Bartholdi D, Rubin BP, Schwab ME. VEGF mRNA induction correlates with changes in the vascular architecture upon spinal cord damage in the rat. Eur J Neurosci. 1997;9:2549–2560. doi: 10.1111/j.1460-9568.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Beggs JL, Waggener JD. Microvascular regeneration following spinal cord injury: the growth sequence and permeability properties of new vessels. Adv Neurol. 1979;22:191–206. [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Minnillo DR, Hagg T, Whittemore SR. Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J Comp Neurol. 2008a;507:1031–1052. doi: 10.1002/cne.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Worth CA, Mahoney ET, Hagg T, Whittemore SR. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008b;28:1771–1785. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Whittemore SR. VEGF165 therapy exacerbates secondary damage following spinal cord injury. Neurochem Res. 2003;28:1693–1703. doi: 10.1023/a:1026013106016. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R, Fujisawa H. The role of glutamate antagonists for the treatment of CNS injury. J Neurotrauma. 1992;9 (Suppl 2):S443–S462. [PubMed] [Google Scholar]
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Weiss HR. Effects of anti-VEGF antibody on blood-brain barrier disruption in focal cerebral ischemia. Exp Neurol. 2007;204:283–287. doi: 10.1016/j.expneurol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ducker TB, Assenmacher DR. Microvascular response to experimental spinal cord trauma. Surg Forum. 1969;20:428–430. [PubMed] [Google Scholar]
- Fernando NT, Koch M, Rothrock C, Gollogly LK, D’Amore PA, Ryeom S, Yoon SS. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14:1529–1539. doi: 10.1158/1078-0432.CCR-07-4126. [DOI] [PubMed] [Google Scholar]
- Fu BM, Shen S. Structural mechanisms of acute VEGF effect on microvessel permeability. Am J Physiol Heart Circ Physiol. 2003;284:H2124–H2135. doi: 10.1152/ajpheart.00894.2002. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato-Kalmar EL, McKinney RA, Schnell L, Rubin BP, Schwab ME. Local changes in vascular architecture following partial spinal cord lesion in the rat. Exp Neurol. 1997;145:322–328. doi: 10.1006/exnr.1997.6449. [DOI] [PubMed] [Google Scholar]
- Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, Minohara M, Murai H, Mihara F, Taniwaki T, Kira Ji. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128:988–1002. doi: 10.1093/brain/awh453. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum JM, Mani N, Rosenstein JM. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp Neurol. 2008;212:108–117. doi: 10.1016/j.expneurol.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, Lennartsson J, Zhu C, Qu Y, Fang C, Hua J, Matsuo O, Fong GH, Ding H, Cao Y, Becker KG, Nash A, Heldin CH, Li X. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996;2:992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- Mire E, Thomasset N, Jakeman LB, Rougon G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol Cell Neurosci. 2008;37:222–235. doi: 10.1016/j.mcn.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Mautes AE, Hall JJ. Characterization of the microvascular glycocalyx in normal and injured spinal cord in the rat. J Comp Neurol. 1996;376:542–556. doi: 10.1002/(SICI)1096-9861(19961223)376:4<542::AID-CNE4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989;482:57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le CJ, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Bono P, Holnthoner W, Chesnes J, Pytowski B, Sihto H, Laakkonen P, Heikkila P, Joensuu H, Alitalo K. VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell. 2008;13:554–556. doi: 10.1016/j.ccr.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin. A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ, Yancopoulos GD. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005;70:411–418. doi: 10.1101/sqb.2005.70.052. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Lima e Silva, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Shah SM, Tatlipinar S, Quinlan E, Sung JU, Tabandeh H, Nguyen QD, Fahmy AS, Zimmer-Galler I, Symons RC, Cedarbaum JM, Campochiaro PA. Dynamic and quantitative analysis of choroidal neovascularization by fluorescein angiography. Invest Ophthalmol Vis Sci. 2006;47:5460–5468. doi: 10.1167/iovs.06-0012. [DOI] [PubMed] [Google Scholar]
- Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des. 2005;11:1353–1389. doi: 10.2174/1381612053507837. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11:219–230. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Skold M, Cullheim S, Hammarberg H, Piehl F, Suneson A, Lake S, Sjogren A, Walum E, Risling M. Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur J Neurosci. 2000;12:3675–3686. doi: 10.1046/j.1460-9568.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Su JL, Chen PS, Chien MH, Chen PB, Chen YH, Lai CC, Hung MC, Kuo ML. Further evidence for expression and function of the VEGF-C/VEGFR-3 axis in cancer cells. Cancer Cell. 2008;13:557–560. doi: 10.1016/j.ccr.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Sun WW, Liu J, Wang XY, Zhang LS, Zhang W, Li LY, Li H, Wang TH. Changes in PDGF expression in spared dorsal root ganglia and associated spinal dorsal horns in cats subjected to partial dorsal root ganglionectomy. Neurosci Lett. 2008;431:112–117. doi: 10.1016/j.neulet.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltar M, Hellström M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Ylä-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, McTigue DM. Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J Comp Neurol. 2008;510:129–144. doi: 10.1002/cne.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero J, Zurita M, de Oya S, Coca S. Vascular endothelial growth/permeability factor in spinal cord injury. J Neurosurg. 1999;90:220–223. doi: 10.3171/spi.1999.90.2.0220. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Guth L. Experimental spinal cord injury: Wallerian degeneration in the dorsal column is followed by revascularization, glial proliferation, and nerve regeneration. Exp Neurol. 1997;147:159–171. doi: 10.1006/exnr.1997.6590. [DOI] [PubMed] [Google Scholar]