Figure 7. RA mechanoreceptors require Ret signaling for their assembly into mechanosensory circuits in the spinal cord and medulla.
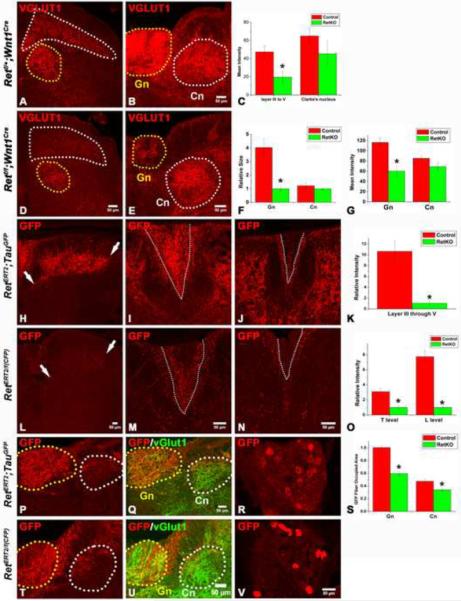
A–B: Synapses of mechanoreceptors, visualized by VGLUT1 staining, in the upper lumbar spinal cord and mid-level medulla of P14 control mice. D–E: Synapses of mechanoreceptors in the upper lumbar spinal cord and mid-level medulla of P14 Retf/f; Wnt1Cre mice. Layers III through V of the spinal cord are outlined by the white dotted line, and Clarke's nucleus is outlined by the yellow dotted line. In the medulla, the gracile nucleus is outlined by the yellow dotted line, and the cuneate nucleus is outlined by the white dotted line. The VGLUT1 staining intensity in layer III through V of spinal cord and gracile nucleus of mutant mice is much lower than that of controls. Quantification of spinal cord staining intensity is shown in panel C (Student's t test, P<0.001 for layers III through V), quantification of the size of the gracile and cuneate nuclei is shown in F (Student's t test, P<0.001 for the gracile nucleus), and quantification of the intensity of the medulla staining is shown in G (Student's t test, P<0.001 for the gracile nucleus). H,L: Anti-GFP staining of RA mechanoreceptor central axonal projections into the spinal cord of P14 RetERT2;Tauf(mGFP) (control) and RetERT/f(CFP) (Ret KO) mice, which were treated with 4-HT at E11.5 and E12.5. Note the reduction in the number of GFP+ axons in RetERT/f(CFP) mice. White arrows indicate the range of projections. Quantification of relative intensity of GFP+ fibers is shown in K. The difference between control and Ret null neurons is statistically significant (Student's t test, P<0.001). I, J: Anti-GFP staining of the dorsal columns of P14 RetERT2;Tauf(mGFP) mice at thoracic (I) and lumbar (J) levels. M,N: Anti-GFP staining of the dorsal columns of P14 RetERT/f(CFP) mice at thoracic (M) and lumbar (N) levels. Many fewer CFP+ fibers are found in the gracile fasciculus of the dorsal column of the mutant (outlined by white dotted lines). Quantification is shown in O. The difference between the control and Ret null neurons is statistically significant (Student's t test, P< 0.001 for the lumbar level, and P<0.01 for the thoracic level). P,Q: Anti-GFP staining of RA mechanosensory fibers innervating the medulla in P14 RetERT2;Tauf(mGFP) mice. T–U: Anti-GFP staining of RA mechanosensory innervation of the medulla in P14 RetERT/f(CFP) mice. The relative area occupied by GFP+ fibers within the gracile (Gn) and cuneat nucleus (Cn) is quantified in S. The difference between the control and Ret null neurons is statistically significant (Student's t test, P<0.01 for the gracile nucleus, and P<0.05 for the cuneate nucleus). R, V: GFP labeled RA mechanosensory DRG neurons in RetERT2;Tauf(mGFP) and RetERT/f(CFP) thoracic DRGs at P14. On average, 8 ± 3 neurons/section are labeled by GFP in RetERT2;Tauf(mGFP) mice while 6 ± 2 neurons/section are labeled by CFP in RetERT/f(CFP) mice. Note that the level of expression of CFP in Ret null neurons is higher than that of control neurons. N=3 for control and Retf/f;Wnt1Cre mice and N=3 for both RetERT2;Tauf(mGFP) and RetERT/f(CFP) mice. 8 to 12 spinal cord sections per animal for each genotype were examined and quantified.
