Abstract
Informed consent was obtained; the study was HIPAA compliant and institutional review board approved. Fourfold accelerated (FFA) two-dimensional (2D) sensitivity encoding (SENSE) (65 seconds) was prospectively compared with its nonaccelerated counterpart (4 minutes 20 seconds) for diagnostic image quality and sharpness of visualization of blood vessels at 1.5 T with three-dimensional (3D) intracranial contrast-enhanced magnetic resonance venography in 18 consecutive volunteers (10 men, eight women; mean age, 48.4 years) and two patients (55-year-old man, 30-year-old woman). Two readers compared FFA 2D SENSE results with results from its nonaccelerated counterpart; they rated visualization of large and medium sinuses as equivalent (P > .1) and that of small deep cerebral veins (P < .01) and superficial cerebral veins (P < .001) as superior. Overall diagnostic image quality ratings were excellent for 62% and 80% of nonaccelerated and FFA 2D SENSE results, respectively (P < .05). FFA 2D SENSE may become the method of choice for fast visualization of intracranial venous vasculature in clinical practice.
Three-dimensional (3D) magnetic resonance (MR) venography frequently is used to image the intracranial vasculature and is useful in the diagnosis of venous sinus thrombosis. In comparison with computed tomography and digital subtraction angiography, the advantages of MR imaging are that it is minimally invasive, it does not involve radiation exposure, and it is performed without iodinated contrast material. Time-of-flight and phase-contrast MR imaging techniques have been used for intracranial venography during the past decade (1–8). Contrast material–enhanced MR venography with gadolinium-based agents has become popular (9,10) and has been regarded as a valuable diagnostic tool that supplements time-of-flight and phase-contrast methods (11–19).
Three-dimensional contrast-enhanced MR venography of the brain requires sub-millimeter spatial resolution for accurate visualization of blood vessels. Since the acquisition volume encompasses the patient’s entire head, a large sampling matrix is needed to provide coverage at the desired resolution. Typically, more than 120 sagittal sections of 1.4-mm partition thickness are acquired, with an average in-plane isotropic resolution of less than 1.0 mm (15). With a repetition time of 6 msec, a 3D contrast-enhanced MR venographic acquisition requires longer than 4 minutes to complete. Although this duration is similar to that of time-of-flight and phase-contrast MR venographic acquisitions with comparable spatial resolution, it is nearly three to four times longer than the acquisition duration for contrast-enhanced MR angiography of other vascular territories. Shorter contrast-enhanced MR venographic acquisition times of 60 seconds have been implemented. The shorter acquisition duration results from a decrease in spatial resolution requirements (13), with the use of MR imagers that have faster gradients and shorter repetition times (18), or from the advantageous use of volumetric interpolated pulse sequences (19).
Sensitivity encoding (SENSE) is a parallel imaging method that can allow reduction of MR acquisition time without the sacrifice of spatial resolution (20–22). The technique has been used to image multiple vascular regions (23,24). The long acquisition time of 3D contrast-enhanced MR venography and the limited temporal window of the first pass of the bolus of contrast material through the intracranial vasculature present an ideal scenario for time-accelerated SENSE imaging. The technical details of the SENSE technique have been described (20–22). We hypothesized that fourfold accelerated (FFA) two-dimensional (2D) SENSE images for 3D contrast-enhanced MR venography are superior to their nonaccelerated counterparts in regard to overall diagnostic image quality and vessel conspicuity. The SENSE images are superior primarily because the imaging time for the FFA 2D SENSE technique is shorter, and this reduction in time leads to a decrease in motion and image blurring. Second, the FFA 2D SENSE data acquisition is completed shortly after the first pass of the bolus of the contrast material, and this completion leads to not only better opacification of vessels in general but also improved depiction of small deep cerebral veins. Thus, the purpose of our study was to prospectively compare FFA 2D SENSE MR imaging with its non-accelerated counterpart for diagnostic image quality and sharpness of the visualization of cerebral veins.
Materials and Methods
Volunteers and Patients
From August 2005 through March 2006, 18 consecutive volunteers (10 men, eight women; mean age, 48.4 years; range, 30–73 years) and two patients (one man, 55 years old; one woman, 30 years old) participated in our prospective study and underwent 3D contrast-enhanced MR venographic examinations. The 55-year-old man had a previous diagnosis of neurosarcoidosis and a subsequent diagnosis of venous sinus thrombosis. The 31-year-old woman had bilateral cerebral venous sinus thromboses in July of 2005 in the setting of factor V Leiden heterozygosity and the use of the birth control patch. Follow-up contrast-enhanced MR venographic examinations for sinus thrombosis were performed in both patients. Our study was Health Insurance Portability and Accountability Act compliant and was approved by the institutional review board; informed consent was obtained from all participants. Subject confidentiality was protected.
MR Examinations
Examinations were performed with 1.5-T MR systems (Excite; GE Medical Systems, Milwaukee, Wis) by using a receive-only eight-element head coil. Subjects were placed supinely, and their heads entered the magnet bore first. Each examination consisted of the following: (a) a series of transverse, sagittal, and coronal localizer images; (b) a 2D transverse single-section timing sequence located just inferior to the skull base for determination of arrival times of the bolus (a small 2-mL bolus of contrast material) in the carotid arteries and jugular veins; (c) a nonaccelerated 3D contrast-enhanced MR venographic acquisition that was considered the standard for comparison; (d) a 3D gradient-echo acquisition to calculate coil sensitivity profiles needed for subsequent SENSE reconstruction (20); and (e) an FFA 2D SENSE 3D contrast-enhanced MR venographic acquisition.
Data collection for both 3D contrast-enhanced MR venographic sequences, as in c and e, was performed with use of the elliptic centric technique (25). Bolus arrival information obtained from b was used to determine the proper start time of the two contrast-enhanced MR venographic acquisitions after administration of 19-mL contrast agent doses. The start time was just prior to contrast material detection in the jugular veins below the skull base. The order in which the two contrast-enhanced MR venographic sequences, as in c and e, were performed was alternated between subjects. The two injections of contrast material were administered approximately 10 minutes apart to allow the contrast material from the first injection of the bolus to clear the vasculature.
Nonaccelerated Contrast-enhanced MR Venography
Nonaccelerated 3D contrast-enhanced MR venography was performed with a fast spoiled gradient-echo pulse sequence. Sagittal sections provided coverage from ear to ear. Imaging was performed with the following parameters: repetition time msec/echo time msec, 6.5/2.6; flip angle, 30°; full echo; bandwidth, 391 Hz/pixel; anteroposterior and superoinferior fields of view (FOVs), 25 cm; sampling matrix, 320 × 320; section thickness, 1.4 mm; and number of sections, 124, yielding a 17.4-cm right-left FOV. The resultant imaging time was 4 minutes 20 seconds, with a voxel resolution of 0.8 × 0.8 × 1.4 mm3.
FFA 2D SENSE Contrast-enhanced MR Venography
With the SENSE technique, the number of acquired samples is reduced in MR imaging (20). In 2D SENSE imaging, this occurs along both the phase- and section-encoding directions (21). This reduction leads to a smaller FOV and aliasing. By using sensitivity information from multiple coils, aliased images can be deterministically unfolded to generate a full FOV representation of the object that is to undergo imaging. Coil sensitivity profiles for each coil element are obtained from a separate calibration acquisition. A 3D gradient-echo acquisition is used for calibration with the following parameters: 10/3; bandwidth, 195 Hz/pixel; flip angle, 10°; full echo; anteroposterior and superoinferior FOVs, 25 cm; sampling matrix, 320 × 160; sagittal section thickness, 2.8 mm; and number of sections, 62.
The FFA 2D SENSE acquisition evaluated in this work shares all parameters with the nonaccelerated acquisition except for the following: reduced anteroposterior FOV, 12.5 cm; sampling matrix, 320 × 160; number of sections, 62; section thickness, 1.4 mm; and reduced right-left FOV, 8.7 cm. The reduction in FOV is intrinsic to FFA 2D SENSE imaging. Because the number of data samples along both the anteroposterior (phase-encoding) and right-left (section-encoding) directions have each been decreased by a factor of two, the imaging time of the 2D SENSE sequence (65 seconds) is only one-quarter of that of the nonaccelerated acquisition time (4 minutes 20 seconds). Images reconstructed from the FFA 2D SENSE acquisition exhibit the same voxel dimension of 0.8 × 0.8 × 1.4 mm3 as images from their nonaccelerated counterpart.
Contrast Material Injection
Gadolinium-based contrast agent (MultiHance; Bracco Diagnostics, Princeton, NJ) and saline were intravenously injected into the antecubital vein in each subject via a commercially available power injector (Spectris; Medrad, Indianola, Pa). For the 2D timing sequence, 2 mL of contrast agent was injected at a rate of 3 mL/sec. For each 3D contrast-enhanced MR venographic acquisition, 19 mL of contrast agent was injected at a rate of 3 mL/sec. Each contrast agent injection was followed by injection of 25 mL of saline at a rate of 2 mL/sec. The same injection protocol was followed for all 20 participants.
Image Processing and Evaluation
Data from both nonaccelerated and FFA 2D SENSE contrast-enhanced MR venographic acquisitions were successfully reconstructed for all examinations. Source images, full-volume and targeted (1–2-cm-thick) maximum intensity projections, and reformatted projections at 6° increments about the superoinferior and right-left axes were transferred to a workstation (Advantage Windows; GE Medical Systems) with 3D volume-viewing software. Two radiologists, one with 16 years (J.H.) and the other with 7 years (N.G.C.) of experience in neurovascular MR imaging, independently assessed the images obtained with both techniques in all 20 participants. Evaluation and assignment of scores were performed in five categories (Table 1) during the course of 1 week after imaging was performed in all 20 participants.
Table 1.
Evaluation Categories for Assessment of Nonaccelerated and FFA 2D SENSE Reconstructions in 20 Study Participants Who Underwent Contrast-enhanced MR Venography
| A: Category 1—Conspicuity and Signal Intensity of Vessels in Five Vessel Groups | |
|---|---|
| Score* | Assessment |
| −2 | SENSE images were markedly inferior to nonaccelerated images |
| −1 | SENSE images were marginally inferior to nonaccelerated images |
| 0 | SENSE images and nonaccelerated images were equivalent |
| 1 | SENSE images were marginally superior to nonaccelerated images |
| 2 | SENSE images were markedly superior to nonaccelerated images |
| B: Category 2—Evaluation of Image Artifacts | |
|---|---|
| Score | Presence, Degree, and Effect on Diagnosis |
| 0 | None |
| 1 | Minor, do not affect diagnosis |
| 2 | Moderate, can potentially affect diagnosis |
| 3 | Severe, probably will interfere with diagnosis or cause image to be nondiagnostic |
| C: Category 3—Signal Intensity of Background Tissue | |
|---|---|
| Score | Presence and Degree |
| 0 | None |
| 1 | Mild, less than 50% of venous signal intensity |
| 2 | Moderate, greater than 50% of venous signal intensity but not approaching venous signal intensity |
| 3 | Intense, approaching venous signal intensity |
| D: Category 4—Arterial Signal Intensity for Circle of Willis and Vertebrobasilar Vessels | |
|---|---|
| Score | Presence and Degree |
| 0 | Absent |
| 1 | Faint, noncontinuous |
| 2 | Moderate, faint and continuous |
| 3 | Intense, approaching or equal to venous signal intensity |
| E: Category 5—Overall Diagnostic Quality of Images | |
|---|---|
| Score | Quality |
| 0 | Nondiagnostic |
| 1 | Marginal |
| 2 | Good |
| 3 | Excellent |
Note.—In category 1, a single comparison score was assigned to each of the five vessel groups. In categories 2–5, a single score was assigned to each acquisition technique (nonaccelerated and FFA 2D SENSE). Scores from two readers were pooled and tested for statistical significance.
The five vessel groups were as follows: group 1—large sinuses, including superior and inferior sagittal, transverse, and sigmoid sinuses and confluence of sinuses (torcular herophili); group 2—medium sinuses and veins, including straight and superior and inferior petrosal sinuses, Rosenthal vein, great cerebral vein of Galen, and internal cerebral vein; group 3—small deep veins, including septal, thalamostriate, and superior and inferior vermian veins; group 4 —superficial veins, including Labbé, Trolard, and cortical veins; and group 5— extracerebral veins, including ophthalamic, paravertebral, and facial veins.
Data from all participants were presented to both readers in a sequential manner. For each participant, results from the acquisition technique associated with the first contrast agent injection were always presented first, followed by those acquired by using the other technique during the second contrast agent injection. Both radiologists did not have knowledge of the order of the contrast agent injection for all participants and were thus blinded to the acquisition technique that was used to generate each data set. Initial raw scores (not shown in this article) only reflected each reader’s preference for the data set associated with either the first or second contrast agent injection in each of the five categories of the evaluation. Another author (H.H.H.), with knowledge of the acquisition techniques and their association with the order of the contrast agent injection in each participant, subsequently translated the raw scores into those shown in Tables 1 and 2.
Table 2.
Summary of Scores Assigned by Two Readers for Categories 2–5
| Category 2, Artifacts* |
Category 3, Background Signal Intensity† |
Category 4, Arterial Signal Intensity‡ |
Category 5, Overall Diagnostic Quality§ |
|||||
|---|---|---|---|---|---|---|---|---|
| Score | Nonaccelerated | SENSE | Injection 1 | Injection 2 | Nonaccelerated | SENSE | Nonaccelerated | SENSE |
| 0 | 33 | 35 | 24 | 0 | 0 | 0 | 0 | 0 |
| 1 | 7 | 4 | 16 | 26 | 13 | 10 | 1 | 0 |
| 2 | 0 | 1 | 0 | 14 | 21 | 21 | 14 | 8 |
| 3 | 0 | 0 | 0 | 0 | 6 | 9 | 25 | 32 |
Note.—See Table 1 for an explanation of the meaning of the scores for each category. Data are number of occurrences for each level of score in each category. Each column of scores sums to 40 (20 studies for reader 1 + 20 studies for reader 2).
P > .1.
P < .001 (significant difference).
P > .05.
P < .05 (significant difference).
In category 1, readers were asked to identify cerebral veins and evaluate their sharpness in visualization and signal intensity. Vessels were classified into five groups on the basis of size and anatomic location. With use of a five-point scale, a comparison score was assigned to each vessel group. Zero scores reflect equal visibility and signal intensity of vessels between the two data sets. Negative and positive scores reflect better visibility and higher signal intensity of vessels in the nonaccelerated and FFA 2D SENSE images, respectively. For categories 2–5, a four-point scale was used to evaluate each of the two acquisition techniques. In category 2, the presence of image artifacts was assessed. If artifacts were observed, readers were asked to comment on the source of the artifacts and whether they affected image interpretation. In category 3, signal intensity of background tissue was evaluated. In category 4, arterial signal intensity in the circle of Willis and vertebrobasilar vessels was assessed. Last, in category 5, the readers assigned a rating for overall diagnostic quality of images.
Statistical Analysis
The Wilcoxon signed rank test and the paired t test were applied individually to scores in each of the five vessel groups in category 1. They were also applied to the differences in scores between the two reviewing radiologists in categories2–5. The ratings from both readers were pooled. The tests were performed to detect any statistically significant differences between nonaccelerated and FFA 2D SENSE ratings. Statistical significance in each comparison was declared if a difference with P < .05 was obtained. A commercially available statistical software package (JMP, version 5.1, 2004; SAS, Cary, NC) was used to perform the calculations.
Results
Category 1
The ratings from both readers have been pooled in Figure 1 such that, for each of the five vessel groups, the sum of occurrences is 40 (20 studies for reader 1 + 20 studies for reader 2). No statistically significant difference was detected for vessel groups 1, 2, and 5. In vessel groups 1 and 2, 68% (27 of 40) and 70% (28 of 40) of the scores were zeros, respectively. This result suggests that the majority of all large dural sinuses and medium-sized cerebral veins were equally visualized with both non-accelerated and FFA 2D SENSE images. For vessel group 5, the distribution of scores was symmetric. Visualization of the extracerebral vessels was preferred for the technique in which the second injection of the bolus of contrast agent was administered, as the time needed for the contrast agent to fill the vessels often is longer than the time needed for the contrast agent to fill vessels in the other four groups. Residual contrast agent from the first injection of the bolus of the contrast agent probably persists and, thus, provides better enhancement during the second injection. Figure 2 shows full-volume sagittal maximum intensity projections in two participants; these maximum intensity projections illustrate comparable vessel visualization and signal intensity of the major sinuses between the two acquisition techniques.
Figure 1.
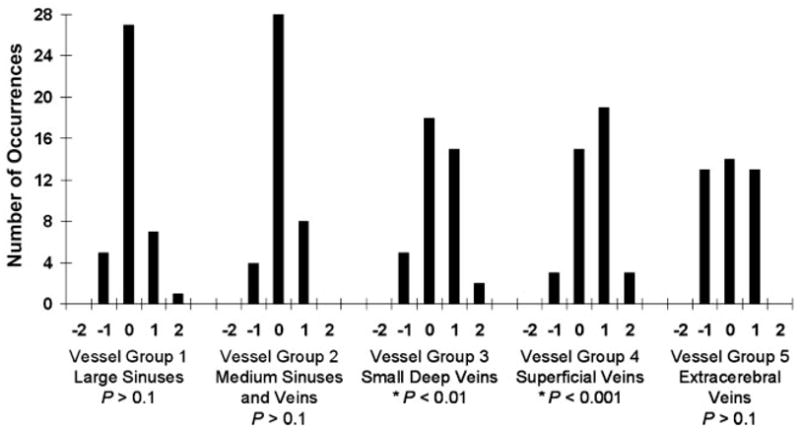
Histogram of pooled scores from two readers for category 1. For each vessel group, the sum of occurrences is 40 (20 studies for reader 1 + 20 studies for reader 2). Numeric scores are defined in Table 1. Positive scores reflect preference for the FFA 2D SENSE results. Negative scores reflect preference for the nonaccelerated acquisition. Statistically significant differences were calculated for each vessel group. FFA 2D SENSE contrast-enhanced MR venography was deemed to be equivalent to nonaccelerated contrast-enhanced MR venography for visualization of vessels in groups 1, 2, and 5 and significantly superior for visualization of vessels in groups 3 and 4.
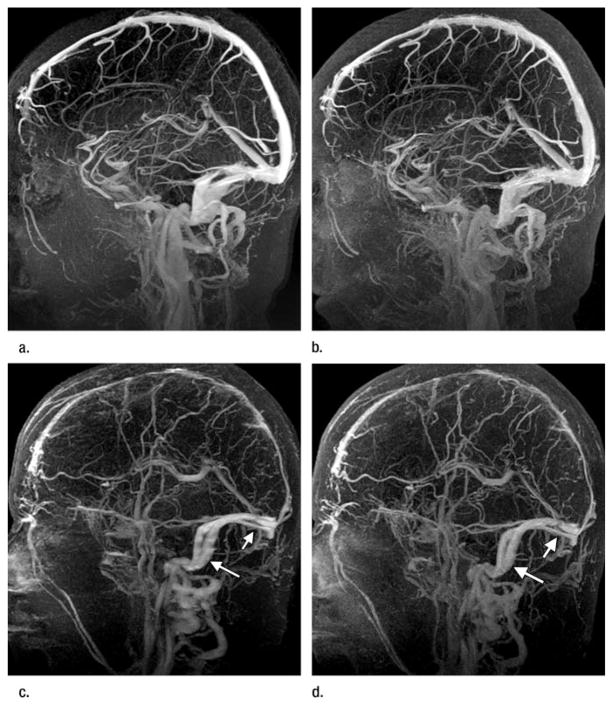
Figure 2.
Three-dimensional contrast-enhanced spoiled gradient-echo MR venographic images (6.5/2.6, 30° flip angle) from (a, b) a healthy volunteer and (c, d) a patient. Sagittal maximum intensity projections from (a, c) nonaccelerated (4 minutes 20 seconds) images show signal intensity and vessel enhancement that are comparable to those in (b, d) corresponding FFA 2D SENSE (65 seconds) acquisitions. All major sinuses and large veins, in addition to smaller vessels including the Labbé and Trolard veins, are equally identified with both techniques. In both studies, the FFA 2D SENSE image was obtained after the second contrast agent injection. Note higher background tissue signal intensity caused by residual contrast agent uptake in the FFA 2D SENSE images. (c, d) MR venographic images in the patient demonstrate changes consistent with sequelae of prior venous thrombosis in the superior sagittal sinus. Note slight blurring of the transverse (short arrow) and sigmoid (long arrow) sinuses in the longer nonaccelerated acquisition in c. This is not observed in the corresponding shorter FFA 2D SENSE image in d. The overall appearance of vessels in d is sharper.
Statistical significance was observed in vessel groups 3 and 4, groups in which the score distributions are right-skewed and show the superiority of the FFA 2D SENSE technique. In group 3, 17 (42%) of 40 positive scores indicated improved visualization of the septal, thalamostriate, and vermian veins with the FFA 2D SENSE technique (P < .01, power = .65). Superiority of the FFA 2D SENSE technique in visualization of small cerebral vessels is further supported by ratings from vessel group 4 (P < .001, power = .98), the group in which 55% (22 of 40) of the scores are positive. Figure 3 illustrates approximate 2-cm-thick targeted projections from reconstructions in two studies and illustrates improved sharpness in delineation of small cerebral veins with the FFA 2D SENSE technique.
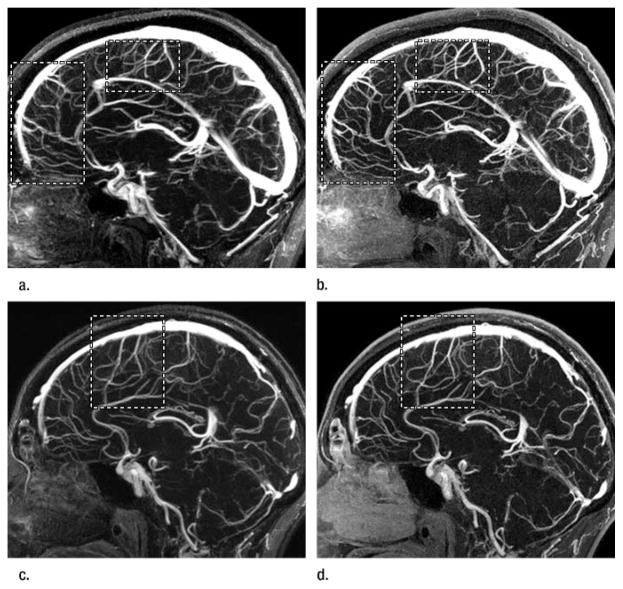
Figure 3.
Sagittal targeted projections of approximately 2 cm thickness containing the superior sagittal sinus from (a, c) nonaccelerated and (b, d) corresponding FFA 2D SENSE reconstructions (6.5/2.6, 30° flip angle) from two studies. In both studies, FFA 2D SENSE images provide superior visualization and sharper delineation of small cerebral vessels defined in vessel groups 3 and 4 in Table 1. The improvement is evident in areas marked with rectangular regions, despite that FFA 2D SENSE images were obtained with the second injection of contrast agent bolus in both studies. Both data sets received positive scores of 1 for vessel groups 3 and 4 from the reviewers.
Categories 2–5
Table 2 summarizes scores for categories 2–5. The scores were pooled for the two readers in Table 2, as was similarly done for the data in Table 1. In categories 2, 4, and 5, the scores reflect a comparison of the two acquisition techniques. Note the exception to category 3 that involved background tissue signal intensity, where ratings are based more appropriately on the order of the injection of the bolus of the contrast agent.
In category 2, there was no significant difference in the level of image artifacts between the nonaccelerated and FFA 2D SENSE results. Ratings of severe artifact levels were not observed for either technique. In one FFA 2D SENSE data set, a score of 2 for a moderate artifact was assigned. This artifact was caused by incomplete SENSE reconstruction as a result of inadequate volume coverage in the calibration image. However, this artifact did not affect image interpretation, and the result was still considered excellent in regard to diagnostic quality of the image. All indications of a minor artifact were attributed to slight image blurring and ghosting. Diagnostic interpretation was not affected by these minor artifacts.
In category 3, a significant difference (P < .001, power = 1.0) was observed in background tissue signal intensity between the first and second injections of the contrast agent. There was a trend toward higher tissue signal intensity for the technique performed after the second injection of the bolus of the contrast agent was administered, and this trend was caused by residual contrast agent uptake from the first injection of the bolus. Figure 4 illustrates representative examples from one participant.
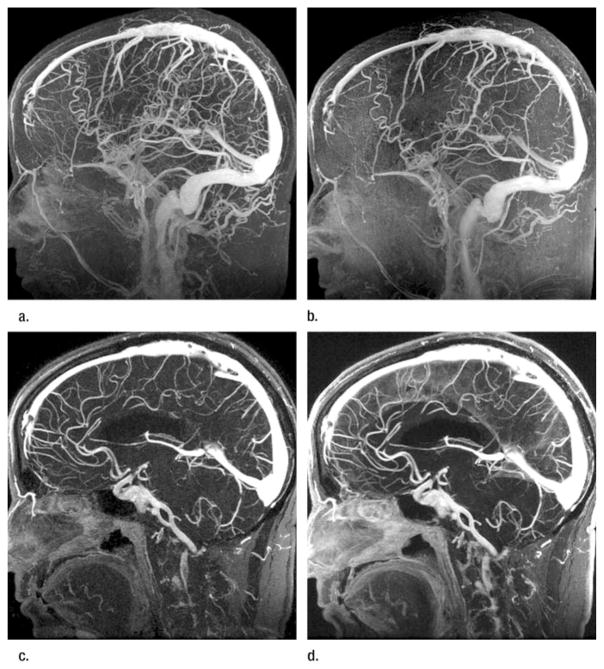
Figure 4.
(a, c) FFA 2D SENSE and (b, d) nonaccelerated images (6.5/2.6, 30° flip angle) in a volunteer. FFA 2D SENSE images were obtained with the first contrast agent injection. Maximum intensity projections of images obtained with the technique performed after the second injection of contrast agent bolus are degraded by increased tissue signal intensity caused by soft-tissue contrast agent uptake from the first injection. Both readers assigned a score of 2 to the images obtained with the nonaccelerated acquisition after the second injection of contrast agent bolus. (b) Sagittal full-volume projection of the data set obtained after the second injection shows obscured visualization of small cerebral veins caused by increased tissue signal intensity. (c, d) Targeted 2-cm-thick projections reveal that vessel enhancement between the two acquisitions is similar.
In category 4, signal intensity in the arteries of the circle of Willis and the vertebrobasilar vascular beds was slightly higher in the FFA 2D SENSE data sets than in the corresponding data sets for the nonaccelerated acquisitions. More than half of the scores in this category reflected moderate to intense contrast-enhanced signal intensity in the arterial system for both nonaccelerated and accelerated acquisition techniques. In all studies, arterial enhancement did not obscure visualization of the venous vasculature.
Scores from category 5 reflect the overall diagnostic quality of the reference and FFA 2D SENSE data sets. Although 62% (25 of 40) of the scores for the nonaccelerated acquisition were of excellent image quality, the FFA 2D SENSE technique was superior for the visualization of the intracranial venous anatomy (P < .05, power = 0.95), with 80% (32 of 40) of the corresponding data evaluated as having excellent diagnostic quality.
Discussion
We demonstrated that FFA 2D SENSE images, compared with nonaccelerated images, obtained with 3D contrast-enhanced MR venography with the same sampling spatial resolution were deemed superior in image quality and vessel sharpness. The 65-second duration of the FFA 2D SENSE acquisition implemented in our study is considerably shorter than the durations with the use of contrast enhancement that were previously reported in the literature (11–13,15). With this method, high spatial resolution is achieved in all three dimensions without volumetric interpolation or partial Fourier techniques (14,19). In addition, all FFA 2D SENSE reconstructions were considered to have sufficient signal intensity for clinical interpretation by the reviewing radiologists, despite the intrinsic loss in signal-to-noise ratio with parallel imaging methods.
Comparisons that we made demonstrated the superiority of the shorter FFA 2D SENSE technique over the non-accelerated method, specifically in the visualization of smaller deep cerebral and superficial cerebral veins. This improvement in vessel visualization is largely caused by the manner of SENSE data sampling, in which, compared with a nonaccelerated image, a greater portion of the elliptic centric k-space is acquired during the first pass of the bolus of the contrast agent through the vasculature. In essence, SENSE sampling increases the effective footprint of the bolus of the contrast agent over k-space and is similar to the use of a larger quantity of the bolus of the contrast agent or a slower contrast agent injection rate. By encoding a larger area in k-space when the transient contrast-enhanced signal is the highest, signal intensity and spatial resolution of small vessels are improved. Although a signal-to-noise ratio loss proportional to √R is typically expected in SENSE imaging when the magnetization is constant during data acquisition and where R is the SENSE acceleration factor, signal-to-noise ratio loss less than a factor of √R can be observed in applications where the magnetization varies with time, such as in contrast-enhanced MR venography (26,27). This phenomenon also has been recently reported in echo-planar imaging (28).
An extension of our work is to incorporate into the FFA 2D SENSE protocol variations in contrast agent quantity and injection rates. Contrast agent quantity and corresponding injection rates used in our study were taken from the standard nonaccelerated acquisition protocol and were not altered during the acquisition of FFA 2D SENSE images. Further work that addresses the contrast agent bolus injection rate and duration could possibly lead to additional image improvement, particularly with regard to the advantage of the shorter 2D SENSE technique. Another direction is to evaluate 2D SENSE 3D contrast-enhanced MR venography with MR imagers that operate at a higher magnetic field strength. For example, the theoretical doubling of signal-to-noise ratio at 3.0 T compared with a similar 1.5-T acquisition can potentially allow 2D SENSE acceleration factors beyond four with a clinically acceptable signal-to-noise ratio. Although images of excellent diagnostic quality were generated with FFA 2D SENSE in this study, greater accelerations in 3D contrast-enhanced MR venography can potentially reduce image time to approximately 45 seconds without a compromise in spatial resolution. Additional 2D SENSE accelerations can also improve the section resolution (right-left dimension) from 1.4 mm to less than 1.0 mm without additional image time, thus allowing the acquired voxel to become truly isotropic.
A limitation of this study is that FFA 2D SENSE 3D contrast-enhanced MR venography has not yet been evaluated as a clinical tool for the detection of venous thrombosis in comparison with nonaccelerated contrast-enhanced MR venography or other time-of-flight and phase-contrast MR imaging techniques. The 18 volunteers in our study population were all considered healthy. Limited data from the two patients are inadequate to draw conclusions about the performance of FFA 2D SENSE contrast-enhanced MR venography in the detection of venous thrombosis.
One of the drawbacks of implementing SENSE is the need to separately acquire coil calibration data with an additional image. Our calibration took 90 seconds to complete and was accommodated by all study participants without a complication or discomfort. However, the additional time required for the acquisition of the calibration image is balanced by the benefits of a shortened contrast-enhanced MR venographic acquisition time, a decreased likelihood of motion and image blurring, and most important, improved visualization of small vessels with SENSE acceleration. The excellent diagnostic quality and superior vessel conspicuity and signal intensity of images obtained with FFA 2D SENSE with 3D contrast-enhanced MR venography have the potential to make this technique the method of choice for fast visualization of the intracranial venous vasculature in clinical practice.
Advances in Knowledge
An acquisition of the intracranial venous system can be completed with submillimeter spatial resolution in approximately 60 seconds with fourfold accelerated (FFA) two-dimensional (2D) sensitivity encoding (SENSE), compared with more than 4 minutes for an acquisition without 2D SENSE with the same sampling spatial resolution.
With use of an eight-element head coil, the FFA 2D SENSE technique yields images of excellent diagnostic quality with high vessel conspicuity and no artifacts.
Contrast-enhanced MR venography with the FFA 2D SENSE technique allowed improved depiction of smaller cerebral vessels.
Implications for Patient Care
Two-dimensional FFA SENSE contrast-enhanced MR venography can provide three-dimensional volumetric coverage of the entire brain with submillimeter spatial resolution in less than 1 minute and has the potential to become the method of choice for fast visualization of the intracranial venous vasculature in clinical practice.
FFA 2D SENSE contrast-enhanced MR venography of the intracranial vasculature helps in not only the reduction of the requisite image time but also the provision of sharper depiction of cerebral vessels in comparison with a non-accelerated acquisition.
The reduced imaging time of FFA 2D SENSE contrast-enhanced MR venography can help to decrease the likelihood of image blurring and artifacts that are typically associated with patient motion.
Acknowledgments
Supported by National Institutes of Health grant EB000212.
The authors thank Kristina M. Schmidtknecht, BA, for her work in volunteer recruitment and study coordination and Roger C. Grimm, MS, for his expertise in MR pulse sequence programming.
Abbreviations
- FFA
fourfold accelerated
- FOV
field of view
- SENSE
sensitivity encoding
- 3D
three-dimensional
- 2D
two-dimensional
Footnotes
Author contributions:
Guarantors of integrity of entire study, H.H.H., S.J.R.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, H.H.H., N.G.C., S.J.R.; clinical studies, all authors; statistical analysis, H.H.H.; and manuscript editing, all authors
Authors stated no financial relationship to disclose.
References
- 1.Edelman RR, Wentz KU, Mattle HP, et al. Intracerebral arteriovenous malformations: evaluation with selective MR angiography and venography. Radiology. 1989;173:831–837. doi: 10.1148/radiology.173.3.2813794. [DOI] [PubMed] [Google Scholar]
- 2.Mattle HP, Wentz KU, Edelman RR, et al. Cerebral venography with MR. Radiology. 1991;178:453–458. doi: 10.1148/radiology.178.2.1987608. [DOI] [PubMed] [Google Scholar]
- 3.Vogl TJ, Bergman C, Villringer A, Einhaupl K, Lissner J, Felix R. Dural sinus thrombosis: value of venous MR angiography for diagnosis and follow-up. AJR Am J Roentgenol. 1994;162:1191–1198. doi: 10.2214/ajr.162.5.8166009. [DOI] [PubMed] [Google Scholar]
- 4.Ozsvath RR, Casey SO, Lustrin ES, Alberico RA, Hassankhani A, Patel M. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169:1699–1707. doi: 10.2214/ajr.169.6.9393193. [DOI] [PubMed] [Google Scholar]
- 5.Liauw L, van Buchem MA, Spilt A, et al. MR angiography of the intracranial venous system. Radiology. 2000;214:678–682. doi: 10.1148/radiology.214.3.r00mr41678. [DOI] [PubMed] [Google Scholar]
- 6.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21:74–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Pui MH. Cerebral MR venography. Clin Imaging. 2004;28:85–89. doi: 10.1016/S0899-7071(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 8.Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol. 2005;252:1021–1025. doi: 10.1007/s00415-005-0710-6. [DOI] [PubMed] [Google Scholar]
- 9.Dormont D, Sag K, Biondi A, Wechsler B, Marsault C. Gadolinium-enhanced MR of chronic dural sinus thrombosis. AJNR Am J Neuroradiol. 1995;16:1347–1352. [PMC free article] [PubMed] [Google Scholar]
- 10.Prince MR, Sostman HD. MR venography: unsung and underutilized. Radiology. 2003;226:630–632. doi: 10.1148/radiol.2263021476. [DOI] [PubMed] [Google Scholar]
- 11.Liang L, Korogi Y, Sugahara T, et al. Evaluation of the intracranial dural sinuses with a 3D contrast-enhanced MP-RAGE sequence: prospective comparison with 2D-TOF MR venography and digital subtraction angiography. AJNR Am J Neuroradiol. 2001;22:481–492. [PMC free article] [PubMed] [Google Scholar]
- 12.Lovblad KO, Schneider J, Bassetti C, et al. Fast contrast-enhanced MR whole-brain venography. Neuroradiology. 2002;44:681–688. doi: 10.1007/s00234-002-0751-9. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof K, Welzel T, Jansen O, Sartor K. More reliable noninvasive visualization of the cerebral veins and dural sinuses: comparison of three MR angiographic techniques. Radiology. 2002;224:804–810. doi: 10.1148/radiol.2243011019. [DOI] [PubMed] [Google Scholar]
- 14.Wetzel SG, Law M, Lee VS, Cha S, Johnson G, Nelson K. Imaging of the intracranial venous system with a contrast-enhanced volumetric interpolated examination. Eur Radiol. 2003;13:1010–1018. doi: 10.1007/s00330-002-1714-6. [DOI] [PubMed] [Google Scholar]
- 15.Farb RI, Scott JN, Willinsky RA, Montanera WJ, Wright GA, terBrugge KG. Intracranial venous system: gadolinium-enhanced three-dimensional MR venography with auto-triggered elliptic centric-ordered sequence—initial experience. Radiology. 2003;226:203–209. doi: 10.1148/radiol.2261020670. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Jung S, Moon KS, et al. Preoperative evaluation of venous systems with 3-dimensional contrast-enhanced magnetic resonance venography in brain tumors: comparison with time-of-flight magnetic resonance venography and digital subtraction angiography. Surg Neurol. 2005;64:128–133. doi: 10.1016/j.surneu.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Rollins N, Ison C, Reyes T, Chia J. Cerebral MR venography in children: comparison of 2D time-of-flight and gadolinium-enhanced 3D gradient-echo techniques. Radiology. 2005;235:1011–1017. doi: 10.1148/radiol.2353041427. [DOI] [PubMed] [Google Scholar]
- 18.Bozzao A, Finocchi V, Romano A, et al. Role of contrast-enhanced MR venography in the preoperative evaluation of parasagittal meningiomas. Eur Radiol. 2005;15:1790–1796. doi: 10.1007/s00330-005-2788-8. [DOI] [PubMed] [Google Scholar]
- 19.Mermuys KP, Vanhoenacker PK, Chappel P, Van Hoe L. Three-dimensional venography of the brain with a volumetric interpolated sequence. Radiology. 2005;234:901–908. doi: 10.1148/radiol.2343031956. [DOI] [PubMed] [Google Scholar]
- 20.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 21.Weiger M, Pruessmann KP, Kassner A, et al. Contrast-enhanced 3D MRA using SENSE. J Magn Reson Imaging. 2000;12:671–677. doi: 10.1002/1522-2586(200011)12:5<671::aid-jmri3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Weiger M, Pruessmann KP, Boesiger P. 2D SENSE for faster 3D MRI. MAGMA. 2002;14:10–19. doi: 10.1007/BF02668182. [DOI] [PubMed] [Google Scholar]
- 23.van den Brink JS, Watanabe Y, Kuhl CK, et al. Implications of SENSE MR in routine clinical practice. Eur J Radiol. 2003;46:3–27. doi: 10.1016/s0720-048x(02)00333-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilson GJ, Hoogeveen RM, Willinek WA, Muthupillai R, Maki JH. Parallel imaging in MR angiography. Top Magn Reson Imaging. 2004;15:169–185. doi: 10.1097/01.rmr.0000134199.94874.70. [DOI] [PubMed] [Google Scholar]
- 25.Wilman AH, Riederer SJ, Huston J, 3rd, Wald JT, Debbins JP. Arterial phase carotid and vertebral artery imaging in 3D contrast-enhanced MR angiography by combining fluoroscopic triggering with an elliptical centric acquisition order. Magn Reson Med. 1998;40:24–35. doi: 10.1002/mrm.1910400104. [DOI] [PubMed] [Google Scholar]
- 26.Fain SB, Riederer SJ, Bernstein MA, Huston J., 3rd Theoretical limits of spatial resolution in elliptical-centric contrast-enhanced 3D-MRA. Magn Reson Med. 1999;42:1106–1116. doi: 10.1002/(sici)1522-2594(199912)42:6<1106::aid-mrm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.Griswold MA, Jakob PM, Chen Q, et al. Resolution enhancement in single-shot imaging using simultaneous acquisition of spatial harmonics (SMASH) Magn Reson Med. 1999;41:1236–1245. doi: 10.1002/(sici)1522-2594(199906)41:6<1236::aid-mrm21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Jaermann T, Pruessmann KP, Valavanis A, Kollias S, Boesiger P. Influence of SENSE on image properties in high-resolution single-shot echo-planar DTI. Magn Reson Med. 2006;55:335–342. doi: 10.1002/mrm.20769. [DOI] [PubMed] [Google Scholar]