Abstract
In BALB/c mice, liver granulomas provoked by visceral infection with intracellular Leishmania donovani are rapidly populated by influxing blood monocytes. To determine the host defense effector role of these mononuclear phagocytes, we treated three populations of infected animals with 5C6, an anti-type 3 complement receptor monoclonal antibody (MAb), which inhibits monocyte recruitment into inflamed tissues. In naive BALB/c mice, injections of 5C6 impaired the initial acquisition of antileishmanial resistance and arrested the development of mature liver granulomas. In sensitized mice with established immunity, both resistance to rechallenge and accelerated granuloma formation were similarly inhibited by MAb administration. Finally, in naive mice, 5C6 MAb also abolished the antileishmanial activity induced by treatment with the macrophage-activating lymphokine gamma interferon. Together, these results suggest a key effector role for the influxing blood monocyte in both initial and established antileishmanial defense and granuloma assembly and in the infected liver as the mononuclear phagocyte target for the antimicrobial effects of gamma interferon.
Full text
PDF
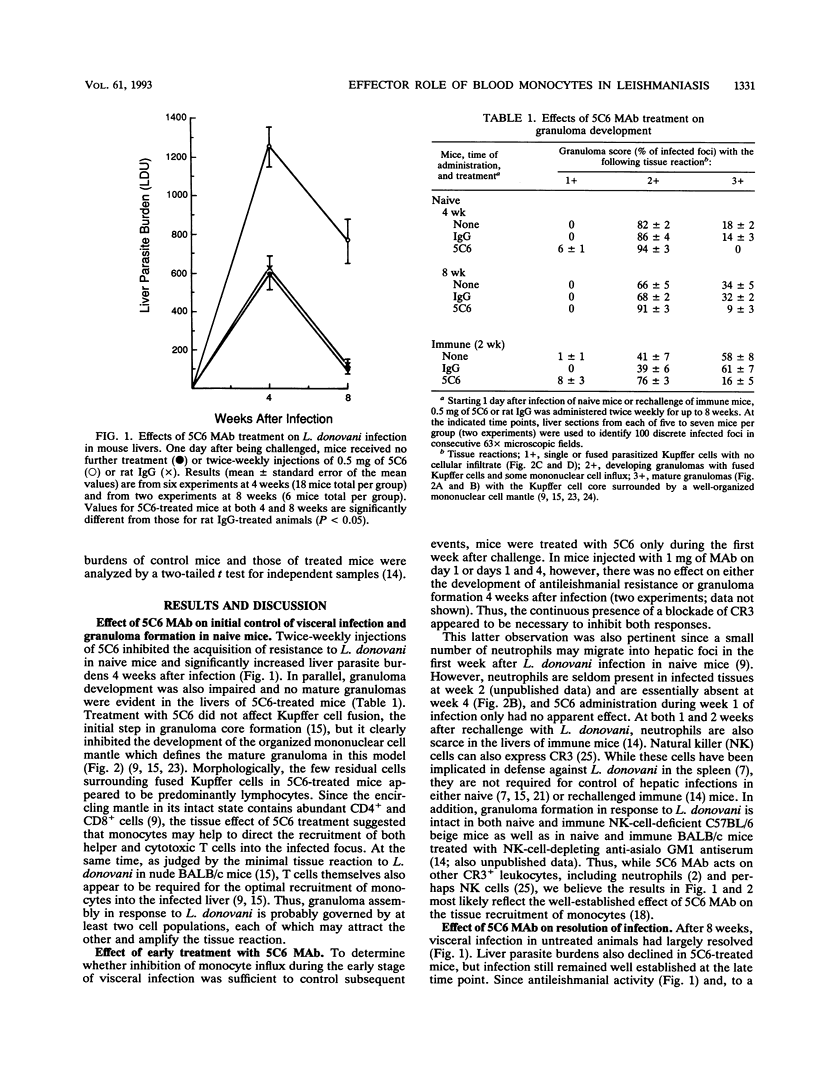
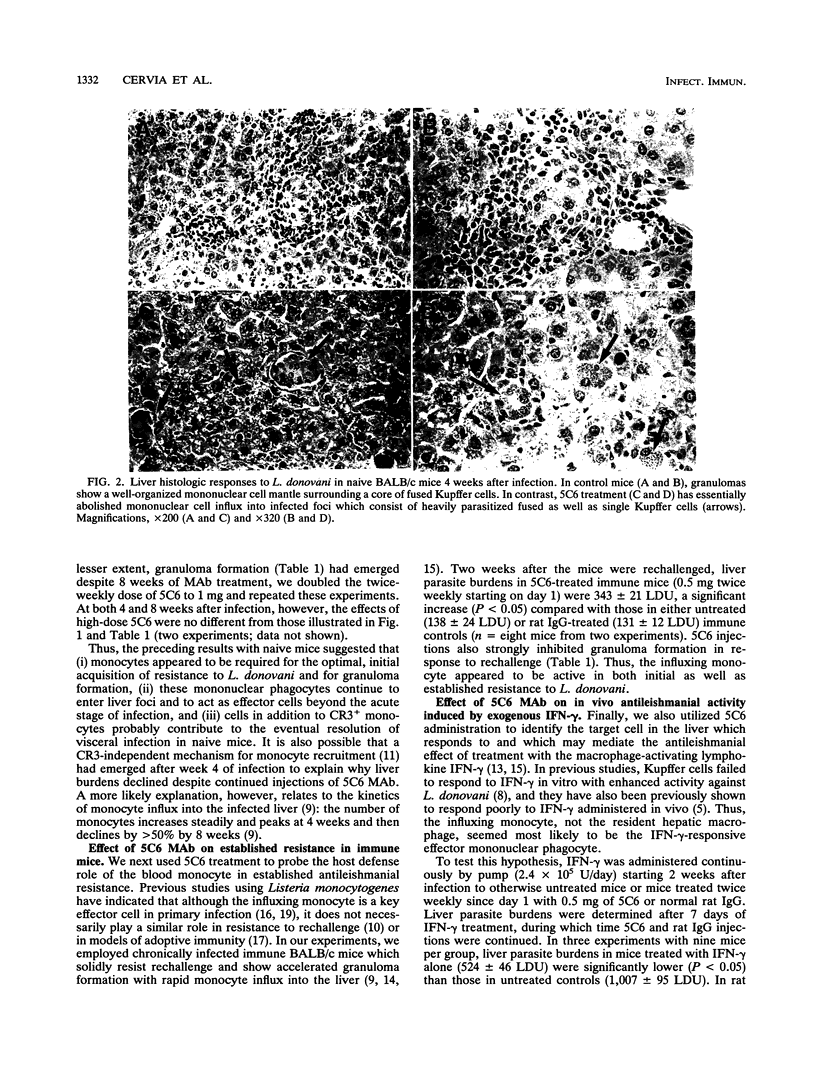

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Rosen H., Blackwell J. M. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988 Dec;65(4):511–514. [PMC free article] [PubMed] [Google Scholar]
- Davies E. V., Singleton A. M., Blackwell J. M. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988 May;56(5):1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A., Nathan C. Analysis of the nonfunctional respiratory burst in murine Kupffer cells. J Exp Med. 1988 Mar 1;167(3):1154–1170. doi: 10.1084/jem.167.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. J., Springer T. A., Thorbecke G. J. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Leishmaniasis in beige mice. Infect Immun. 1982 Dec;38(3):1208–1216. doi: 10.1128/iai.38.3.1208-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Rosen H., Brocke S., Peters C., Hahn H. Protective immunity and granuloma formation are mediated by two distinct tumor necrosis factor alpha- and gamma interferon-dependent T cell-phagocyte interactions in murine listeriosis: dissociation on the basis of phagocyte adhesion mechanisms. Infect Immun. 1992 May;60(5):1875–1882. doi: 10.1128/iai.60.5.1875-1882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovich A. M., Galelli A., Allison A. C., Modabber F. Z. Increased myelopoiesis during Leishmania major infection in mice: generation of 'safe targets', a possible way to evade the effector immune mechanism. Clin Exp Immunol. 1986 Apr;64(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Effect of continuous administration of interferon-gamma in experimental visceral leishmaniasis. J Infect Dis. 1990 May;161(5):992–994. doi: 10.1093/infdis/161.5.992. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Squires K. E., Miralles C. D., Stoeckle M. Y., Granger A. M., Granelli-Piperno A., Bogdan C. Acquired resistance and granuloma formation in experimental visceral leishmaniasis. Differential T cell and lymphokine roles in initial versus established immunity. J Immunol. 1992 Mar 15;148(6):1858–1863. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. C., Demartini J. C., Orme I. M. Passive transfer of acquired resistance to Listeria monocytogenes infection is independent of mononuclear cell granuloma formation. Infect Immun. 1987 Dec;55(12):3215–3218. doi: 10.1128/iai.55.12.3215-3218.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. Role of CR3 in induced myelomonocytic recruitment: insights from in vivo monoclonal antibody studies in the mouse. J Leukoc Biol. 1990 Nov;48(5):465–469. doi: 10.1002/jlb.48.5.465. [DOI] [PubMed] [Google Scholar]
- Smith L. E., Rodrigues M., Russell D. G. The interaction between CD8+ cytotoxic T cells and Leishmania-infected macrophages. J Exp Med. 1991 Sep 1;174(3):499–505. doi: 10.1084/jem.174.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires K. E., Kirsch M., Silverstein S. C., Acosta A., McElrath M. J., Murray H. W. Defect in the tissue cellular immune response: experimental visceral leishmaniasis in euthymic C57BL/6 ep/ep mice. Infect Immun. 1990 Dec;58(12):3893–3898. doi: 10.1128/iai.58.12.3893-3898.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Wiltrout R. H., Mathieson B. J., Talmadge J. E., Reynolds C. W., Zhang S. R., Herberman R. B., Ortaldo J. R. Augmentation of organ-associated natural killer activity by biological response modifiers. Isolation and characterization of large granular lymphocytes from the liver. J Exp Med. 1984 Nov 1;160(5):1431–1449. doi: 10.1084/jem.160.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]



