Abstract
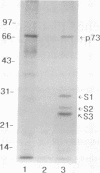
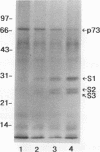
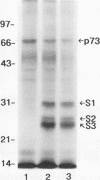
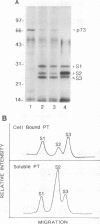
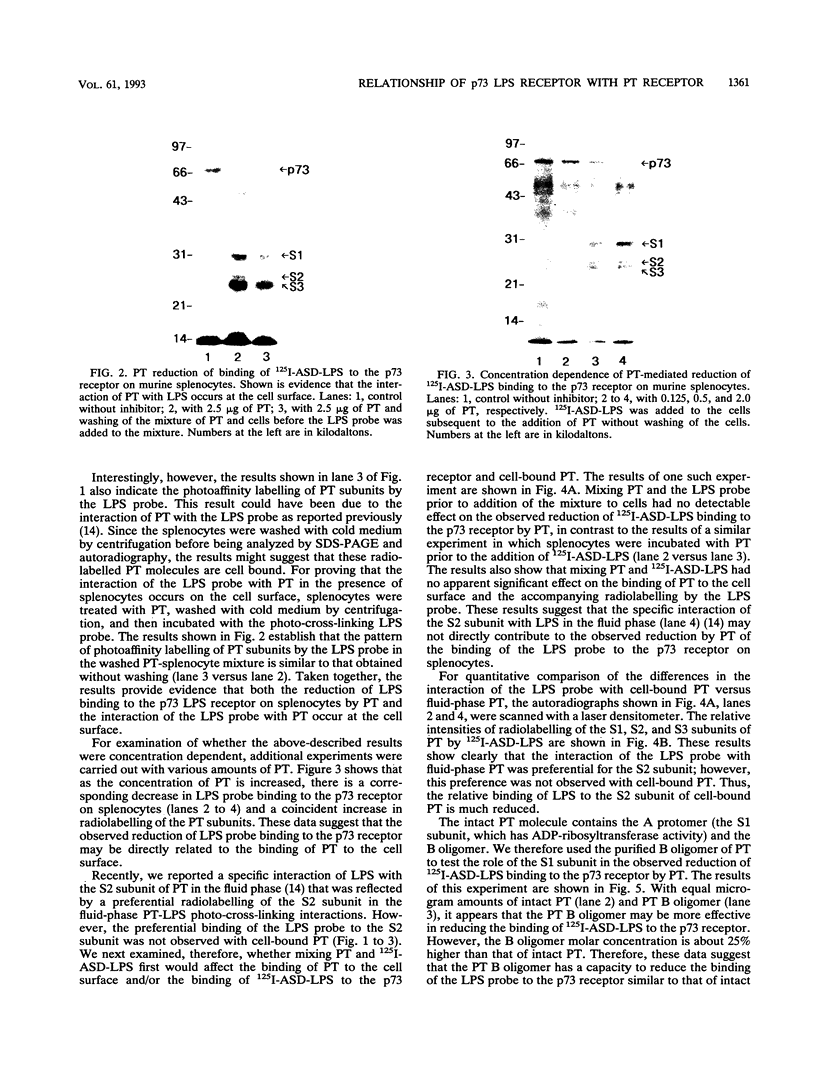
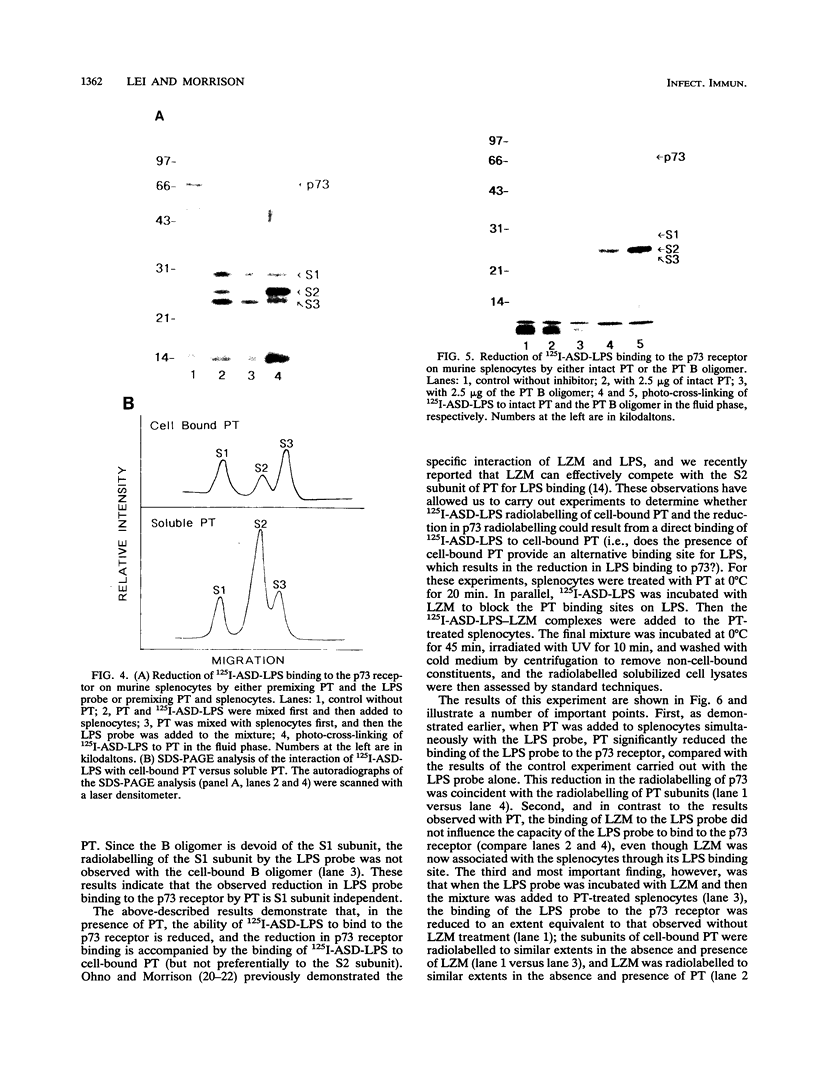
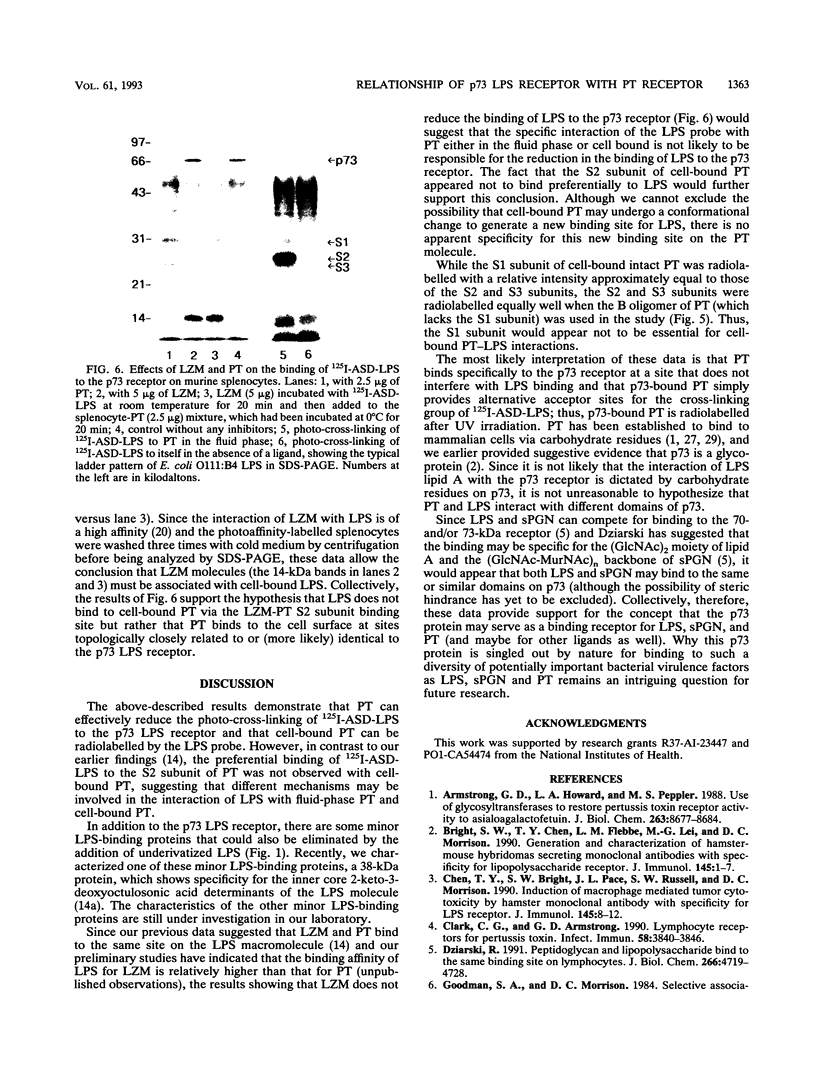
In previous studies, we used a photoactivable, radioiodinated lipopolysaccharide (LPS) derivative to define and characterize a specific bacterial endotoxic LPS-binding protein (p73) on mammalian lymphoreticular cells, including B and T lymphocytes and macrophages. More recently, using the same methodology, we characterized a specific interaction of LPS with the S2 subunit of Bordetella pertussis pertussis toxin (PT) in the fluid phase (M.-G. Lei and D. C. Morrison, J. Biol. Chem., 268:1488-1493, 1993). Furthermore, we showed that lysozyme (LZM) but not polymyxin B can compete with PT for binding to LPS in the fluid phase, a result suggesting that these two molecules compete for the same binding site on LPS. In this report, we demonstrate that the binding of PT to murine splenocytes (cell-bound PT) reduces the ability of the LPS photo-cross-linking probe to bind to the p73 receptor. The reduction can also be demonstrated with the PT B oligomer, a result indicating that the observed reduction of LPS binding to the p73 receptor by PT is A-protomer (S1-subunit) independent. More importantly, our studies document that cell-bound PT can be radiolabelled by the LPS probe, coincident with the observed reduction in p73 photoaffinity labelling. The preferential interaction of LPS with the PT S2 subunit in the fluid phase was, however, not observed with cell-bound PT. The reduction in radiolabelling of the p73 receptor by the LPS probe and in radiolabelling of cell-bound PT was shown to be concentration dependent. The data presented here document, however, that LZM does not reduce the ability of the LPS probe to bind to the p73 receptor on mouse splenocytes, nor does the presence of LZM bound to LPS influence the observed reduction in photoaffinity labelling of p73 by the LPS probe or radiolabelling of cell-bound PT by the LPS probe. Collectively, these results support the concept that the ability of LPS to interact with PT in the fluid phase is not responsible for the ability of cell-bound PT to influence the binding of the LPS probe to the p73 receptor. Thus, it is suggested that PT and LPS bind to different sites on the p73 molecule and that this same p73 protein may recognize both LPS and PT.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. D., Howard L. A., Peppler M. S. Use of glycosyltransferases to restore pertussis toxin receptor activity to asialoagalactofetuin. J Biol Chem. 1988 Jun 25;263(18):8677–8684. [PubMed] [Google Scholar]
- Bright S. W., Chen T. Y., Flebbe L. M., Lei M. G., Morrison D. C. Generation and characterization of hamster-mouse hybridomas secreting monoclonal antibodies with specificity for lipopolysaccharide receptor. J Immunol. 1990 Jul 1;145(1):1–7. [PubMed] [Google Scholar]
- Chen T. Y., Bright S. W., Pace J. L., Russell S. W., Morrison D. C. Induction of macrophage-mediated tumor cytotoxicity by a hamster monoclonal antibody with specificity for lipopolysaccharide receptor. J Immunol. 1990 Jul 1;145(1):8–12. [PubMed] [Google Scholar]
- Clark C. G., Armstrong G. D. Lymphocyte receptors for pertussis toxin. Infect Immun. 1990 Dec;58(12):3840–3846. doi: 10.1128/iai.58.12.3840-3846.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Peptidoglycan and lipopolysaccharide bind to the same binding site on lymphocytes. J Biol Chem. 1991 Mar 15;266(8):4719–4725. [PubMed] [Google Scholar]
- Goodman S. A., Morrison D. C. Selective association of lipid-rich R-like lipopolysaccharide subunits with murine spleen cells. Mol Immunol. 1984 Aug;21(8):689–697. doi: 10.1016/0161-5890(84)90021-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Chen T. Y., Crawford R. M., Nacy C. A., Morrison D. C., Meltzer M. S. Cytotoxic activity and production of toxic nitrogen oxides by macrophages treated with IFN-gamma and monoclonal antibodies against the 73-kDa lipopolysaccharide receptor. J Immunol. 1992 Sep 15;149(6):2069–2075. [PubMed] [Google Scholar]
- Halling J. L., Hamill D. R., Lei M. G., Morrison D. C. Identification and characterization of lipopolysaccharide-binding proteins on human peripheral blood cell populations. Infect Immun. 1992 Mar;60(3):845–852. doi: 10.1128/iai.60.3.845-852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge S., Amano F., Nishijima M., Akamatsu Y. Isolation of a lipopolysaccharide (LPS)-resistant mutant, with defective LPS binding, of cultured macrophage-like cells. J Biol Chem. 1990 Apr 25;265(12):6606–6610. [PubMed] [Google Scholar]
- Kirkland T. N., Virca G. D., Kuus-Reichel T., Multer F. K., Kim S. Y., Ulevitch R. J., Tobias P. S. Identification of lipopolysaccharide-binding proteins in 70Z/3 cells by photoaffinity cross-linking. J Biol Chem. 1990 Jun 5;265(16):9520–9525. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Lipopolysaccharide interaction with S2 subunit of pertussis toxin. J Biol Chem. 1993 Jan 15;268(2):1488–1493. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- Lei M. G., Stimpson S. A., Morrison D. C. Specific endotoxic lipopolysaccharide-binding receptors on murine splenocytes. III. Binding specificity and characterization. J Immunol. 1991 Sep 15;147(6):1925–1932. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Morrison D. C., Silverstein R., Bright S. W., Chen T. Y., Flebbe L. M., Lei M. G. Monoclonal antibody to mouse lipopolysaccharide receptor protects mice against the lethal effects of endotoxin. J Infect Dis. 1990 Nov;162(5):1063–1068. doi: 10.1093/infdis/162.5.1063. [DOI] [PubMed] [Google Scholar]
- Ohno N., Morrison D. C. Effects of lipopolysaccharide chemotype structure on binding and inactivation of hen egg lysozyme. Eur J Biochem. 1989 Dec 22;186(3):621–627. doi: 10.1111/j.1432-1033.1989.tb15252.x. [DOI] [PubMed] [Google Scholar]
- Ohno N., Morrison D. C. Lipopolysaccharide interaction with lysozyme. Binding of lipopolysaccharide to lysozyme and inhibition of lysozyme enzymatic activity. J Biol Chem. 1989 Mar 15;264(8):4434–4441. [PubMed] [Google Scholar]
- Ohno N., Morrison D. C. Lipopolysaccharide interactions with lysozyme differentially affect lipopolysaccharide immunostimulatory activity. Eur J Biochem. 1989 Dec 22;186(3):629–636. doi: 10.1111/j.1432-1033.1989.tb15253.x. [DOI] [PubMed] [Google Scholar]
- Roeder D. J., Lei M. G., Morrison D. C. Endotoxic-lipopolysaccharide-specific binding proteins on lymphoid cells of various animal species: association with endotoxin susceptibility. Infect Immun. 1989 Apr;57(4):1054–1058. doi: 10.1128/iai.57.4.1054-1058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Witvliet M. H., Burns D. L., Brennan M. J., Poolman J. T., Manclark C. R. Binding of pertussis toxin to eucaryotic cells and glycoproteins. Infect Immun. 1989 Nov;57(11):3324–3330. doi: 10.1128/iai.57.11.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J Immunol. 1993 Feb 1;150(3):1011–1018. [PubMed] [Google Scholar]
- van't Wout J., Burnette W. N., Mar V. L., Rozdzinski E., Wright S. D., Tuomanen E. I. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect Immun. 1992 Aug;60(8):3303–3308. doi: 10.1128/iai.60.8.3303-3308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]