Table 1.
A General Synthesis of 2-Amido-Furans.
| entry | ynamides | 2-amido-furans | yield [%]:a | using Ab | Bc |
|---|---|---|---|---|---|
| 1 | 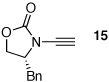 |
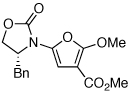 |
18 | 82 | 44 |
| 2 | 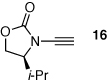 |
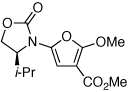 |
19 | 65 | 48 |
| 3 | 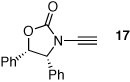 |
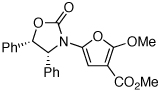 |
20 | 77 | 48 |
| 4 | 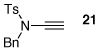 |
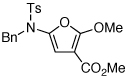 |
25 | 50 | 24 |
| 5 | 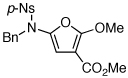 |
26 | 47 | 29 | |
| 6 | 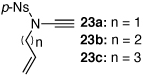 |
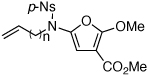 |
27a | 49 | 32 |
| 7 | 27b | 58 | 35 | ||
| 8 | 27c | 61 | 47 | ||
| 9 | 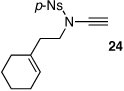 |
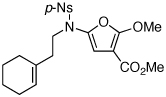 |
28 | 52 | 39 |
| 10 | 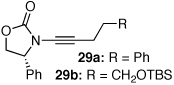 |
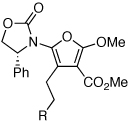 |
30a | 45d | 37e |
| 11 | 30b | 24d | 22e |
Isolated yields.
When using A, all reactions were run in toluene at 80 °C with 2 mol % Rh2(OAc)4, and ynamide concn = 0.15 M. Reagent A [3.0 equiv] was delivered over 1–2 h as a solution in toluene [concn = 0.30 M] via a syringe pump.
When using B, all reactions were run in CH2Cl2 at rt for 1.5 h with 5 mol % Rh2(OAc)4 and 4Å MS, and ynamide concn = 0.15 M. Reagent B [2.0 equiv] was delivered as solid in 0.5 equiv portions over 1 h.
4.0 equiv of A and 5 mol % Rh2(OAc)4 were used, and temp was 110 °C.
Reactions were carried out in ClCH2CH2Cl [concn = 0.15 M] at 50 °C, and a total of 4.0 equiv of reagent B was used.
