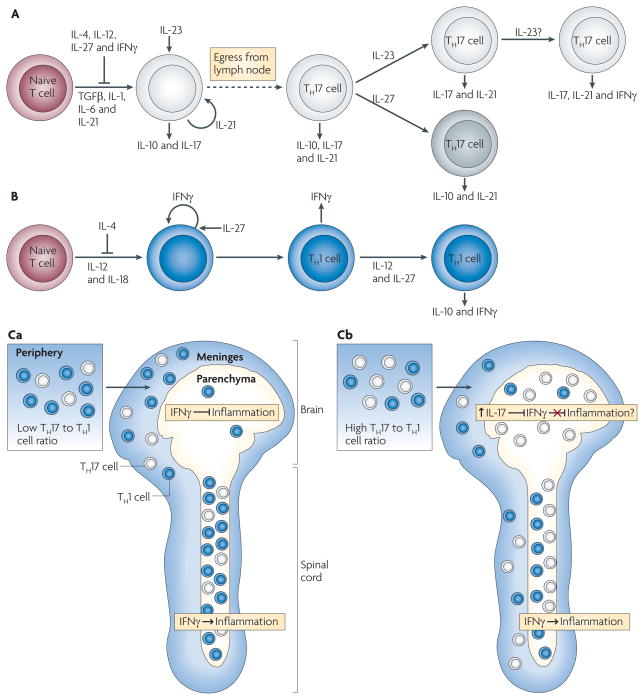
Figure 3. Model of TH1 and TH17 cell differentiation.
A. | Naive T cells differentiate in the presence of transforming growth factor-β (TGFβ), interleukin-1 (IL-1), IL-6 and IL-21 into an early T helper 17 (TH17) cell stage that also produces IL-10 and IL-17. IL-4, IL-12, IL-27 and interferon-γ (IFNγ) inhibit TH17 cell differentiation, and IL-21 acts in an autocrine manner at this stage. IL-23 promotes TH17 cell differentiation, decreases IL-10 production and stabilizes the IL-17-producing phenotype. IL-23 may facilitate co-expression of IFNγ under some circumstances. IL-27 can suppress IL-17 and induce IL-10 production by TH17 cells. B | Differentiation of TH1 cells occurs in the presence of IL-12 and IL-18, with IFNγ acting in an autocrine manner. TH1 cell differentiation is inhibited by IL-4, and IL-27 can enhance IFNγ production by differentiating TH1 cells. Exposure to IL-12 and IL-27 induces differentiated TH1 cells to produce IL-10 and IFNγ. Differentiated TH17 and TH1 cells express other pro-inflammatory cytokines (not depicted). Ca | Myelin-specific T cells with a low TH17 to TH1 cell ratio are primed in the periphery and infiltrate the meninges of both the brain and spinal cord. The T cells enter the spinal cord parenchyma and induce inflammation in an IFNγ-mediated signalling-dependent manner. The few T cells that enter the brain parenchyma, where IFNγ-mediated signalling inhibits inflammation, do not recruit inflammatory cells. Cb | Myelin-specific T cells primed in the periphery with a high TH17 to TH1 cell ratio infiltrate the meninges and parenchyma of both the brain and spinal cord. The higher TH17 to TH1 cell ratio in the brain is associated with a disproportionate increase in IL-17 production compared with the spinal cord, which may overcome the inhibitory IFNγ-mediated signalling in the brain parenchyma.