Figure 1.
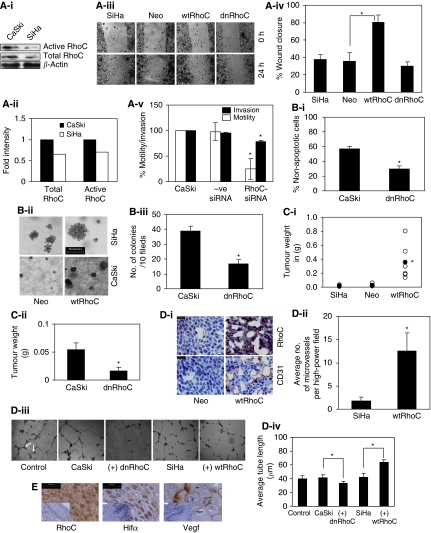
RhoC drives tumour progression in cervical carcinoma. (A-i) Cell extracts were analysed for RhoC expression in CaSki and SiHa cells. The SiHa cells show less RhoC expression and activity compared with CaSki cells (n=2). (A-ii) Graphical presentation of panel Ai. (A-iii) Representative images showing wtRhoC expression in SiHa cells increased motility in a wound-healing assay (magnification × 40). (A-iv) Graphical presentation of panel A-iii showing faster wound closure by SiHa-wtRhoC compared with SiHa-Neo (P<0.05; n=4). (A-v) RhoC-siRNA reduced motility (P<0.01) and invasion (P<0.02) of CaSki cells, –ve siRNA did not alter motility and invasion of CaSki cells. CaSki cells were transfected with siRNA for 48 h, cells were trypsinised and seeded for a transwell migration assay (n=3). (B-i) Graphical presentation of the effect of dnRhoC resistance to anoikis. Expression of dnRhoC led to decreased CaSki cell survival (30% non-apoptotic cells; P<0.001) cultured for 7 h under anoikis (n=3). (B-ii) CaSki and SiHa cells formed large colonies upon wtRhoC overexpression compared with parental cells. Cells were transiently transfected with wtRhoC, cultured for 48 h and were seeded for a clonogenic assay (n=3). (B-iii) Graphical representation of the effect of dnRhoC on colony formation. The expression of dnRhoC resulted in lesser number of colonies (16/10 fields; P<0.003) compared with parental CaSki cells (n=3). (C-i) Overexpression of wtRhoC in SiHa cells resulted in tumours 15-folds heavier (P<0.02) than SiHa tumours. The black-filled circles indicate the mean tumour weight for each tumour type (n=6). The empty circles indicate individual tumour weights (n=6). (C-ii) Expression of dnRhoC in CaSki cells resulted in smaller tumours, average weight 0.04 g (P<0.019) in xenograft assays in nude mice (n=3). (D-i) Xenograft cryosections were stained for RhoC and CD31. Representative images suggest increased RhoC and microvessel-specific CD31 expression in SiHa-wtRhoC tumours. (D-ii) Graphical representation of microvessel density in SiHa-wtRhoC xenografts. Expression of wtRhoC formed more blood vessels, ∼12.5 (P<0.009) microvessels per high-power field compared with SiHa tumours. The lumen with CD31 expression was counted in 10 high-power fields from at least four different sections. (D-iii) Illustrative images representing the effect of RhoC on tube formation by human umbilical vascular endothelial cells (HUVECs). A total of 7000 cells were seeded in 60% Matrigel (BD Biosciences) and cultured for 16 h with indicated conditioned medium. The distance between the two arrows indicates the length of the tubes (magnification × 200). (D-iv) Ectopic expression of RhoC and its mutant altered in vitro tube formation by HUVECs in Matrigel. Conditioned media from CaSki-dnRhoC and SiHa-wtRhoC, respectively, decreased and increased tube formation (P<0.001; n=3). (E) Cervical carcinoma tissue sections immunohistochemically assessed for expression of RhoC, Hifα and Vegf in serial sections of same tumour sample (scale bar=30 μm). Corresponding normal tissue section images presented as inset. *Significant difference between the data set.