Abstract
Objective
Evaluation of the capacity of lower thoracic spinal cord stimulation (SCS) to activate the expiratory muscles and generate large airway pressures and high peak airflows characteristic of cough, in subjects with tetraplegia.
Design
Clinical trial.
Setting
In-patient hospital setting for electrode insertion; out-patient setting for measurement of respiratory pressures; home setting for application of SCS.
Participants
Subjects (N = 9; 8 men, 1 woman) with cervical spinal cord injury and weak cough.
Intervention(s)
A fully implantable electrical stimulation system was surgically placed in each subject. Partial hemilaminectomies were made to place single-disc electrodes in the epidural space at the T9, T11 and L1 spinal levels. A radiofrequency receiver was placed in the subcutaneous pocket over the anterior portion of the chest wall. Electrode wires were tunneled subcutaneously and connected to the receiver. Stimulation was applied by activating a small portable external stimulus controller box powered by a rechargeable battery to each electrode lead alone and in combination.
Main Outcome Measure(s)
Airway pressure and peak airflow generation achieved with SCS.
Results
Supramaximal SCS resulted in large airway pressures and high peak airflow rates during stimulation at each electrode lead. Maximum airway pressures and peak airflow rates were achieved with combined stimulation of any 2 leads. At total lung capacity, mean maximum airway pressure generation and peak airflow rates were 137 ± 30 cmH2O (mean ± SE) and 8.6 ± 1.8 (mean ± SE) L/s, respectively.
Conclusions
Lower thoracic SCS results in near maximum activation of the expiratory muscles and the generation of high positive airway pressures and peak airflow rates in the range of those observed with maximum cough efforts in normal individuals.
Keywords: Electric Stimulation, Quadriplegia, Cough, Respiratory Muscles, Spinal Cord Injuries
Introduction
Cervical and high thoracic spinal cord injury results in paralysis of the expiratory intercostal and abdominal muscles, the major muscle groups responsible for generating the high positive airway pressures characteristic of a normal cough (1–3). The ability of subjects with spinal cord injury (SCI) to clear airway secretions therefore is markedly impaired resulting in physical discomfort, inconvenience and the development of atelectasis and recurrent respiratory tract infections (4–8). Consequently, these subjects are dependent upon caregiver assistance for the application of manual suctioning, assisted coughing maneuvers or other methods of airway management (9–12). These techniques are generally uncomfortable, cumbersome and often restrict patient mobility. Moreover, despite their use, respiratory tract infections remain a major cause of morbidity and mortality in this patient population (13–16).
In theory, restoration of expiratory muscle function and thereby an effective cough mechanism, would improve life quality, enhance mobility, eliminate the need for artificial methods of secretion clearance and potentially reduce the incidence of respiratory complications in SCI. Since the neuromuscular apparatus below the level of injury is generally intact, the expiratory muscles are amenable to a variety of stimulation techniques (3, 17–21). Based upon previous investigations in animals (18, 22, 23), we hypothesized that lower thoracic spinal cord stimulation (SCS) would result in the generation of large airway pressures and high peak flow rates in SCI, characteristic of a normal cough.
In this study, we present the results of a clinical trial in which lower thoracic SCS was applied to activate the expiratory muscles in SCI. By this technique, single-disc electrodes are positioned in the dorsal epidural space at the lower thoracic and upper lumbar spinal levels. In this paper, the capacity of this technique to activate the expiratory muscles and generate large positive airway pressures and high peak flow rates is presented. The stimulus-output relationships and effects of stimulation of different electrode combinations are also described. In a companion paper (24), we report the clinical effects of lower thoracic SCS in terms of benefits, risks and side effects. Preliminary results of this technique were described previously in a case report (25).
Methods
This investigation was approved by the Institutional Review Board, the National Institute of Neurological Disorders and Stroke and the Food and Drug Administration. Informed consent was obtained from each subject before enrollment in the study.
All research subjects suffered from some form of traumatic injury to their cervical spinal cord and were in stable condition at the time of study entrance. None of the subjects suffered from significant lung, cardiac or brain disease, which represent exclusion criteria. Among the inclusion criteria for study subjects were objective evidence of expiratory muscle weakness and symptoms of an inadequate cough. Each subject had significant paresis of their expiratory muscles as evidenced by markedly reduced maximum expiratory pressures and peak expiratory flow rates less than 30 cmH2O and 2.5 L/s, respectively, measured at total lung capacity (TLC). In addition, all subjects complained of difficulty coughing and mobilizing secretions.
Electrical Stimulation System
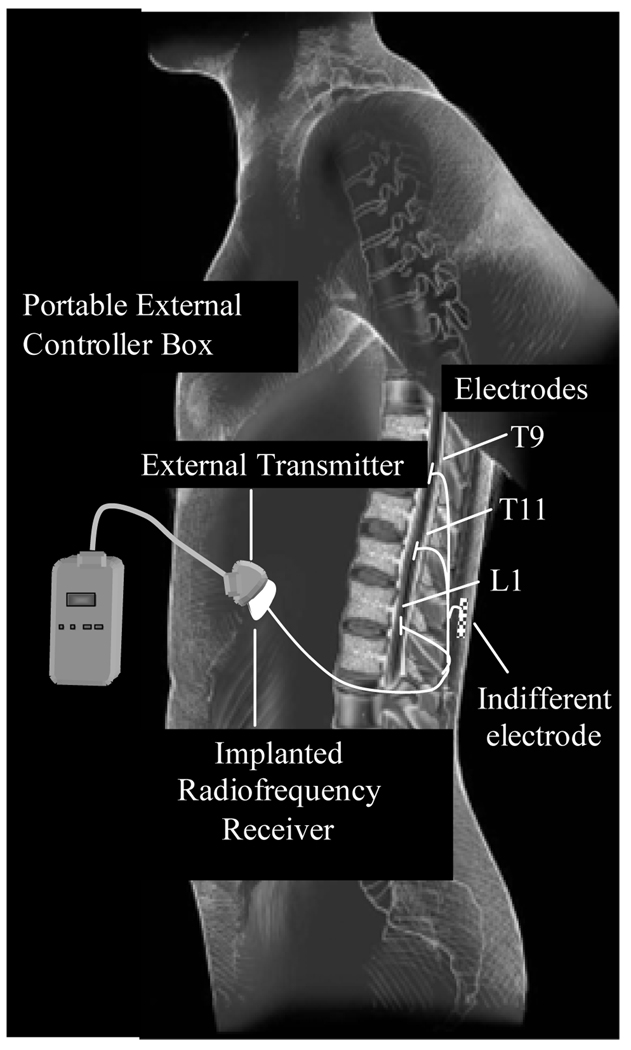
In a single procedure, a fully implantable electrical stimulation system was surgically placed in each subject to activate the expiratory muscles. Each subject underwent semi hemi laminotomies to place three, 4 mm single-lead, platinum-iridium disc electrodes1 at the T9, T11 and L1 spinal levels (Figure 1). Electrodes were positioned in the midline in the epidural space overlying the thecal sac using fluoroscopic guidance. A single-disc, ground electrode (30 mm) was placed under the surface of the thoracolumbar fascia, A radiofrequency receiver (7.6 × 4.6 × 0.85 cm; 12 g)2 was placed in a subcutaneous pocket over the anterior portion of the chest wall, either over the lower rib cage or upper abdominal wall. The electrode wires were tunneled subcutaneously and connected to the receiver. During electrical stimulation applied in the operating room, contraction of the expiratory muscles was confirmed by visual inspection and palpation of the chest wall.
Figure 1.
Electrical Stimulation System. See text for further explanation.
Post-operatively, stimulation was applied by activating a small portable external control box (9.5 × 6 × 2.5 cm) connected to a rubberized transmitter, which was secured to the skin with tape directly over the implanted receiver. The stimulus controller box, which is powered by a rechargeable battery, delivers a radiofrequency signal to the implanted receiver, which is converted to an electrical signal that is transmitted to the electrodes (Figure 1). The stimulator provides a biphasic stimulus over a wide range of stimulus amplitudes (10–40 V), stimulus frequencies (2–105 Hz) and pulse widths (16–800 µs). Stimulus on-time could be adjusted between 0.2 and 50 s.
Muscle Reconditioning
Prior to use of the cough system, 2–3 weeks were allowed to elapse to provide time for regression of edema and hemorrhage at the electrode and receiver sites and healing of all wounds. It was assumed that the expiratory muscles were significantly atrophied secondary to disuse and would require a period of repeated muscle stimulation to restore strength. After an initial evaluation session, subjects were instructed to apply stimulation every 30 s for 5–10 min, 2 or 3 times/day, in the home or nursing home setting. Stimulus parameters were set at values resulting in near maximal positive airway pressure generation, as tolerated, since high intensity force generation for short periods results in the greatest increases in muscles strength (26–28). Subjects were also instructed to use the device for evacuation of secretions or pharyngeal clearance, as needed.
Measurements
Airway pressure was monitored with a pressure transducer3 to assess the force of expiratory muscle contraction. Expiratory flow rates were monitored by use of a heated pneumotachograph4. Measurements were made with use of a tight fitting full face mask or through tracheostomy tube, when present. Subjects with tracheostomies all had cuffless tubes. Dressings were applied around the tracheostomy, therefore, to minimize air leak. In the seated posture, airway pressure measurements were made under conditions of airway occlusion at functional residual capacity (FRC) and total lung capacity (TLC). Cheek pressure was maintained manually during SCS. In a separate maneuver, peak expiratory airflow was measured following release of airway occlusion after peak airway pressure was achieved during SCS. Pressure and flow measurements were recorded on an 8-channel recorder5. During the period of stimulation, subjects were instructed to relax completely. In instances of subject effort, evidence of glottic closure or obvious mask leakage, data were discarded.
During the initial phase of stimulation, blood pressure, pulse rate and oxygen saturation6 were closely monitored. If absolute blood pressure exceeded 140 mmHg systolic or 100 mmHg diastolic, stimulation was withheld until values returned to baseline or below 140 mmHg systolic and 90 mmHg diastolic. Stimulation was then applied at less frequent intervals.
Repeat measurements were made during out-patient visits every 4–5 weeks during the first 28 weeks, then at 3 month intervals for 6 months, and then at 6 month intervals. The inspiratory capacity was assessed at each subject visit as an index of resting lung volume.
Following 2–3 months of daily application of stimulation, the potential for expiratory muscle fatigue was assessed. Airway pressure generation was measured during maximum stimulation, applied every minute for a 30 min period. A decline in pressure generation of 20% or greater was arbitrarily taken as evidence of muscle fatigue.
Results
The specific clinical characteristics of the 9 spinal cord injured subjects are provided in Table 1. The interval between the time of injury and study entry ranged between 1 and 34 years. The spontaneous vital capacities of each subject were variably reduced ranging from 11 to 47% predicted (Table 1).
Table 1.
Clinical Data of the Subjects
| Subject | Sex | Age (y) |
Cause of Injury |
Level of Injury |
Elapsed Time Since Injury (y) |
Spontaneous Vital Capacity (L) (% predicted) |
Maximal Expiratory Pressure (cmH2O) (% predicted) |
Peak Expiratory Flow (L/s) (% predicted) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | MVA | C5/C6 | 7 | 1.96 (39%) | 21 (10%) | 2.1 (21%) |
| 2 | F | 28 | MVA | C3 | 22 | 0.36 (11%) | 16 (10%) | 0.7 (12%) |
| 3 | M | 42 | GSW | C4 | 19 | 1.22 (29%) | 21 (10%) | 1.4 (16%) |
| 4 | M | 28 | Sport | C4/C5 | 2 | 2.64 (45%) | 26 (11%) | 2.2 (21%) |
| 5 | M | 50 | Violence | C5/C6 | 12 | 2.40 (47%) | 24 (11%) | 2.0 (21%) |
| 6 | M | 23 | Diving | C4/C5 | 1 | 0.90 (17%) | 24 (11%) | 1.8 (19%) |
| 7 | M | 45 | Trampoline | C5/C6 | 2 | 1.40 (23%) | 20 (9%) | 2.1 (20%) |
| 8 | M | 49 | Diving | C3/C4 | 34 | 1.70 (30%) | 22 (10%) | 2.0 (18%) |
| 9 | M | 52 | MVA | C3 | 19 | 1.70 (30%) | 28 (13%) | 2.4 (23%) |
MVA – Motor Vehicle Accident
GSW – Gunshot Wound
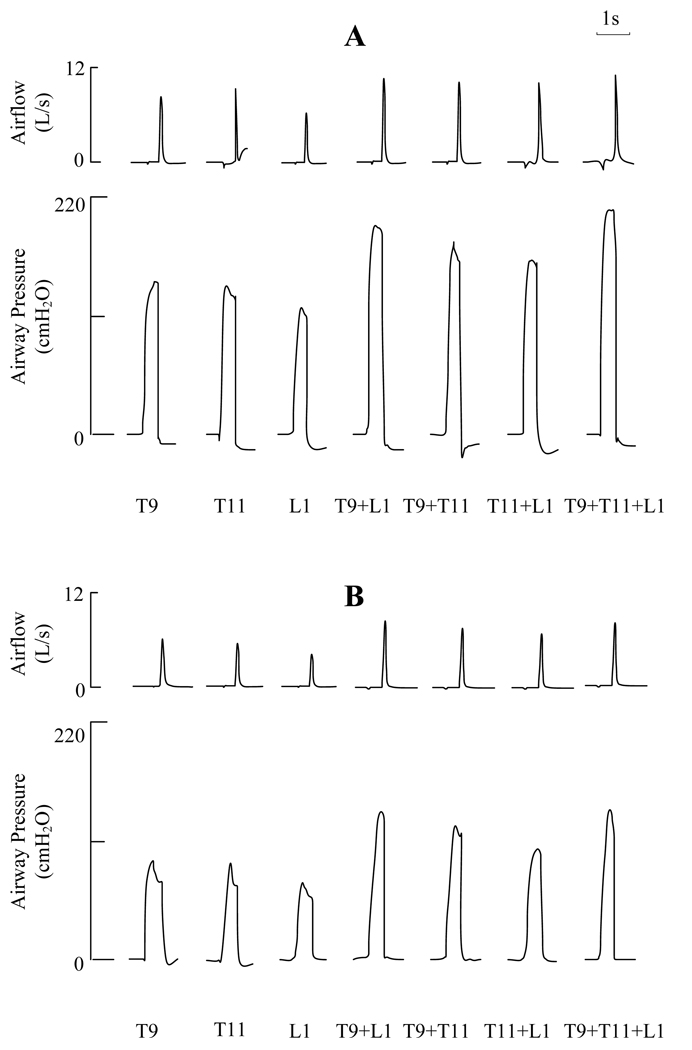
Utilizing maximum stimulus parameters, the effects of lower thoracic SCS at the T9, T11 and L1 spinal levels alone and during combined stimulation at T9 + L1, T9 + T11, T11 + L1 and combined stimulation of all 3 electrodes, on airflow rates and airway pressure generation are shown for one subject in Figure 2. The effects of stimulation at TLC and FRC are shown in panels A and B, respectively. Stimulation at each individual site alone resulted in large airway pressures and high peak flow rates in the range of 120–144 cmH2O and 5.8–8.6 L/s at TLC, respectively. Combined stimulation with any 2 electrode combination resulted in substantially greater values in the range of 162–206 cmH2O and 10.1–10.6 L/s at TLC, respectively. Stimulation with 3 electrodes, however, resulted in no significant increases in these parameters. As expected, peak flow rate and airway pressure generation were smaller at FRC compared to TLC during single and multi-site stimulation. Nonetheless, these values were still substantial. Combined stimulation with 2 electrodes, for example, resulted in airway pressures and peak flow rates in the range of 107–134 cmH2O and 6.7–7.7 L/s.
Figure 2.
Effects of lower thoracic SCS for one subject on airflow and airway pressure generation during stimulation at the T9, T11 and L1 spinal levels alone and in combinations at TLC (panel A) and at FRC (panel B). Large airway pressures and airflow rates were generated during single site stimulation. These parameters were greater with combined stimulation at any 2 sites. Combined stimulation at all 3 sites did not result in further increases in these parameters. See text for further explanation.
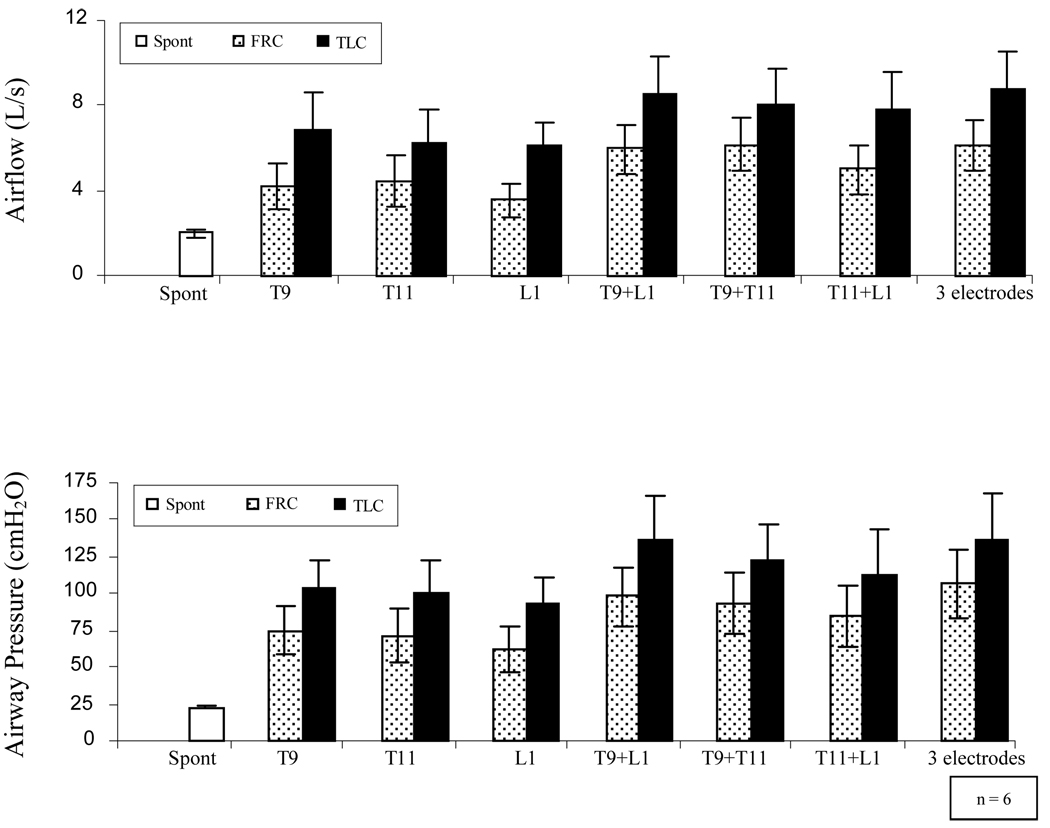
The mean changes in airway pressure generation and peak airflow rates at these same stimulation sites are provided in Figure 3. During stimulation at individual spinal levels, the mean changes in airway pressure at TLC ranged between 94 and 105 cmH2O while the mean peak flow rates ranged between 6.1 and 6.9 L/s. While qualitatively similar results were observed during SCS at FRC, the magnitude of airway pressures and peak flow rates were significantly smaller. During single site stimulation at FRC, mean airway pressure generation ranged between 62 and 75 cmH2O while peak flow rates ranged between 3.6 and 4.5 L/s (p < 0.05 compared to each FRC value). There were no significant differences in airway pressure or peak flow rate generation between individual sites, either at FRC or TLC.
Figure 3.
Mean peak airflow rates (upper panel) and mean airway pressures (lower panel) during SCS at the T9, T11, and L1 spinal levels alone and in combinations at TLC (solid bars) and at FRC (dotted bars). Mean spontaneous airway pressure and peak expiratory flow rates are shown for comparison (empty bars). Large airway pressures and peak airflow rates of similar magnitude were generated during SCS during single site stimulation. Combined stimulation of 2 sites, however, resulted in significantly greater airway pressures and peak airflow rates (p < 0.05, for each). There were no significant differences in either peak airflow rates or airway pressure generation between any 2 sites. Combined stimulation of 3 sites did not result in further increases in these parameters. See text for further explanation.
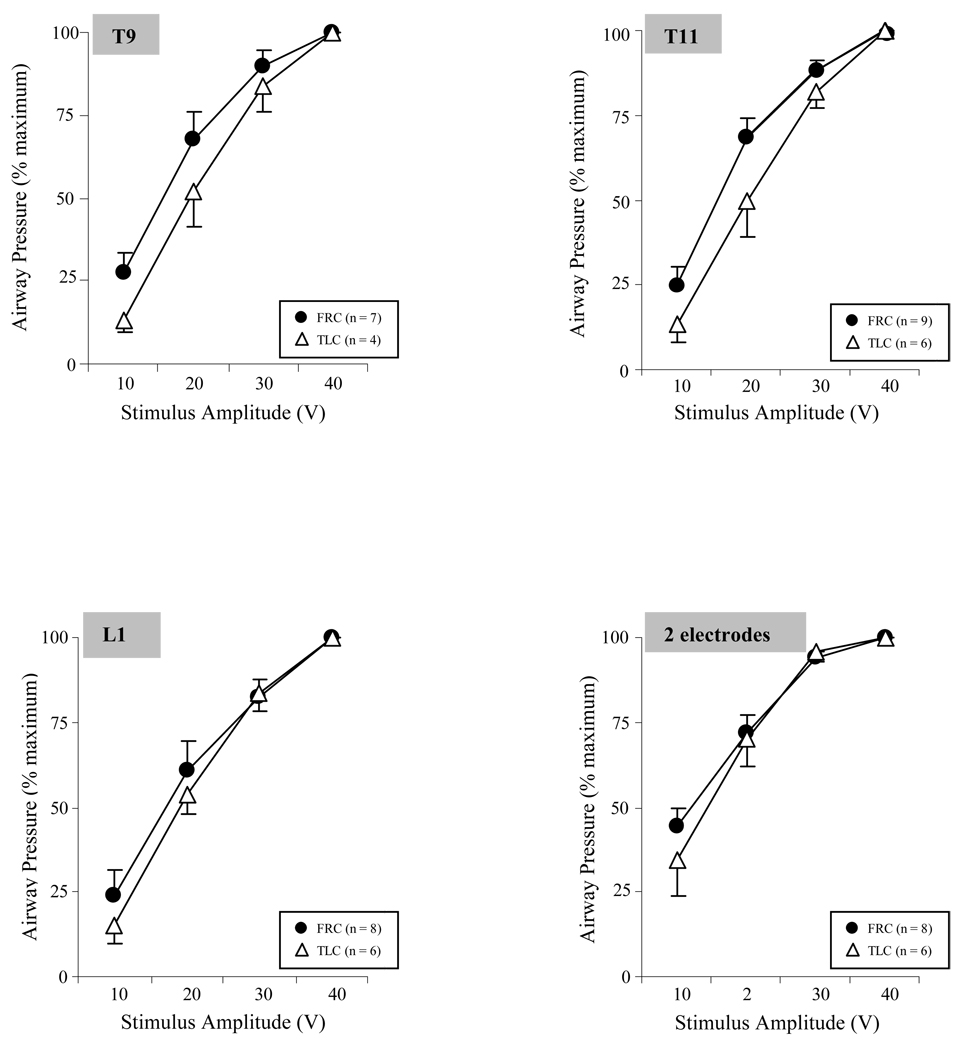
SCS at 2 sites resulted in significant increases in airway pressure and peak flow rate generation to 124–150 cmH2O and 7.8–8.8 L/s, respectively at TLC and to 85–98 cmH2O and 5.0–6.2 L/s, respectively at FRC. These values were significantly greater than those achieved with any single site stimulation alone (p < 0.05). The effects of SCS at all 3 sites in combination, however, were not significantly different than those achieved with SCS at 2 sites, either at TLC or FRC (p > 0.05). There were no significant differences between any of the 2 site combinations in terms of peak airflow or airway pressure generation at TLC or FRC.
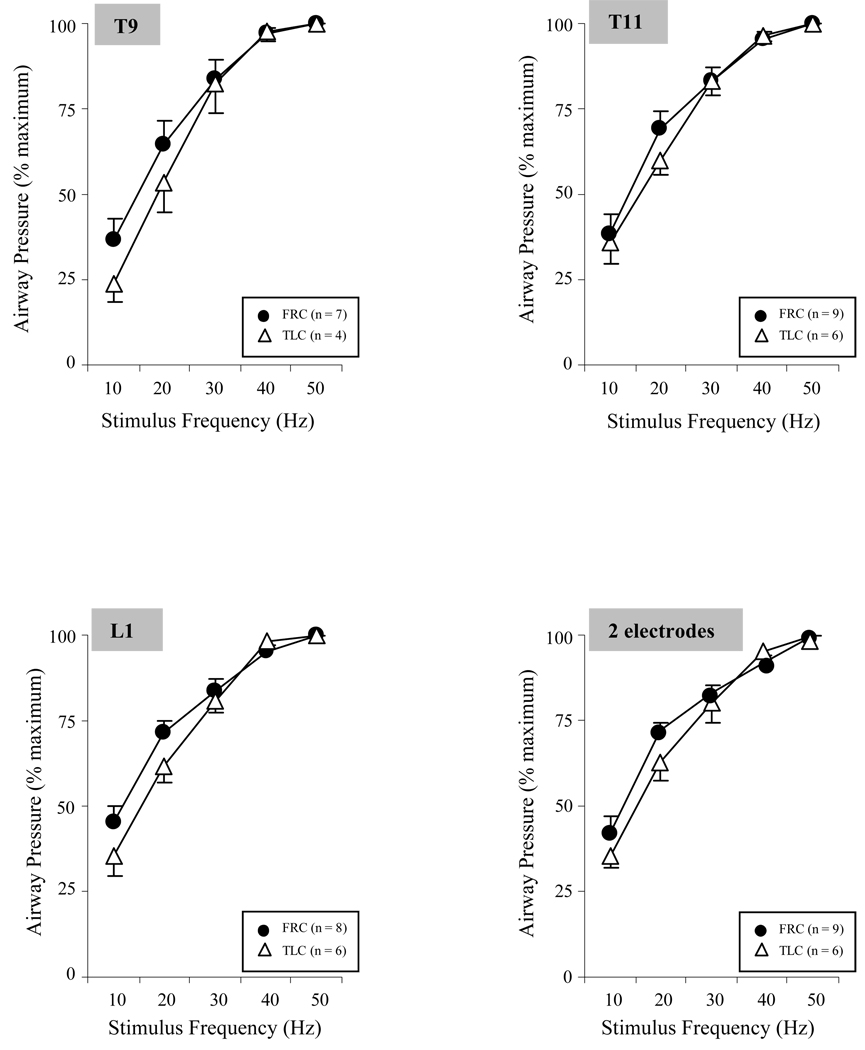
The relationships between stimulus frequency (10–50 Hz) and airway pressure generation (at maximal stimulus amplitude and pulse width of 200 µs) at TLC and FRC, expressed as a percentage of control values, is shown in Figure 4. With increases in stimulus frequency, there were significant increases in airway pressure generation during both single site stimulation and also with a 2 electrode combination. A plateau in pressure generation developed between 40 and 50 Hz.
Figure 4.
Relationship between stimulus frequency (Hz) and mean airway pressure generation (expressed as a percent maximum) during single site SCS and combined stimulation of 2 sites at FRC and at TLC. There were progressive increases in airway pressure generation with increases in stimulus frequency. There was a plateau between 40 and 50 Hz, as there were only small changes in pressure generation between these stimulus frequencies. There were no significant differences between responses at TLC and FRC. See text for further explanation.
The relationships between stimulus amplitude and airway pressure generation (stimulus frequency of 50 Hz and pulse width of 200 µs) at TLC and FRC, expressed as a percentage of control values, is shown in Figure 5. During stimulation at each individual site, there were progressive increases in airway pressure with increasing stimulus amplitude with no apparent plateau in pressure generation. With the 2 electrode combination, there were also progressive increases in pressure generation with increasing stimulus amplitude. However, there were no significant differences between 30 and 40 V (p > 0.05) suggesting the development of a plateau in pressure generation at these amplitude levels.
Figure 5.
Relationship between stimulus amplitude (V) and mean airway pressure generation (expressed as a percent maximum) during single site SCS and combined stimulation of 2 sites at FRC and at TLC. There were progressive increases in airway pressure generation with increasing stimulus amplitude. With 2 site stimulation, a plateau developed between 30 and 40 V, as there were no significant differences in pressure generation between these amplitude levels (p > 0.05). There were no significant differences between responses at TLC and FRC. See text for further explanation.
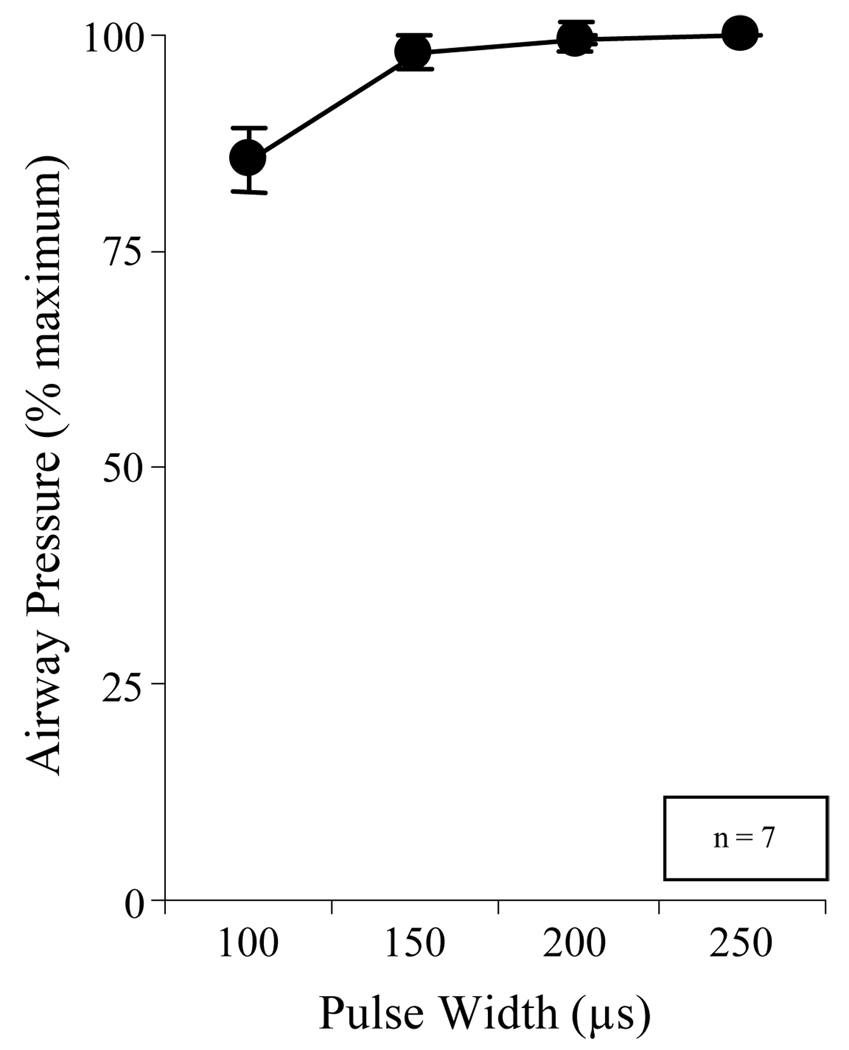
The relationship between pulse width and airway pressure generation (at 40 V and 50 Hz) is shown in Figure 6. Mean airway pressure generation did not increase with the application of pulse widths exceeding 150 µs.
Figure 6.
Relationship between pulse width (µs) and mean airway pressure generation (expressed as a percent maximum) during combined stimulation at 2 sites at TLC. There was a significant increase in pressure generation between 100 and 150 µs (p < 0.05). However, there were no further increases in pressure generation with increasing pulse duration as high as 250 µs.
Based upon these measurements, supramaximal stimulus parameters, i.e. stimulus frequency, amplitude and pulse width values above which there were no further significant increases in pressure generation, were determined for each subject. While there was some variation between subjects, supramaximal parameters ranged between 30 and 40 V, 30–40 Hz with a pulse width of 150–200 µs.
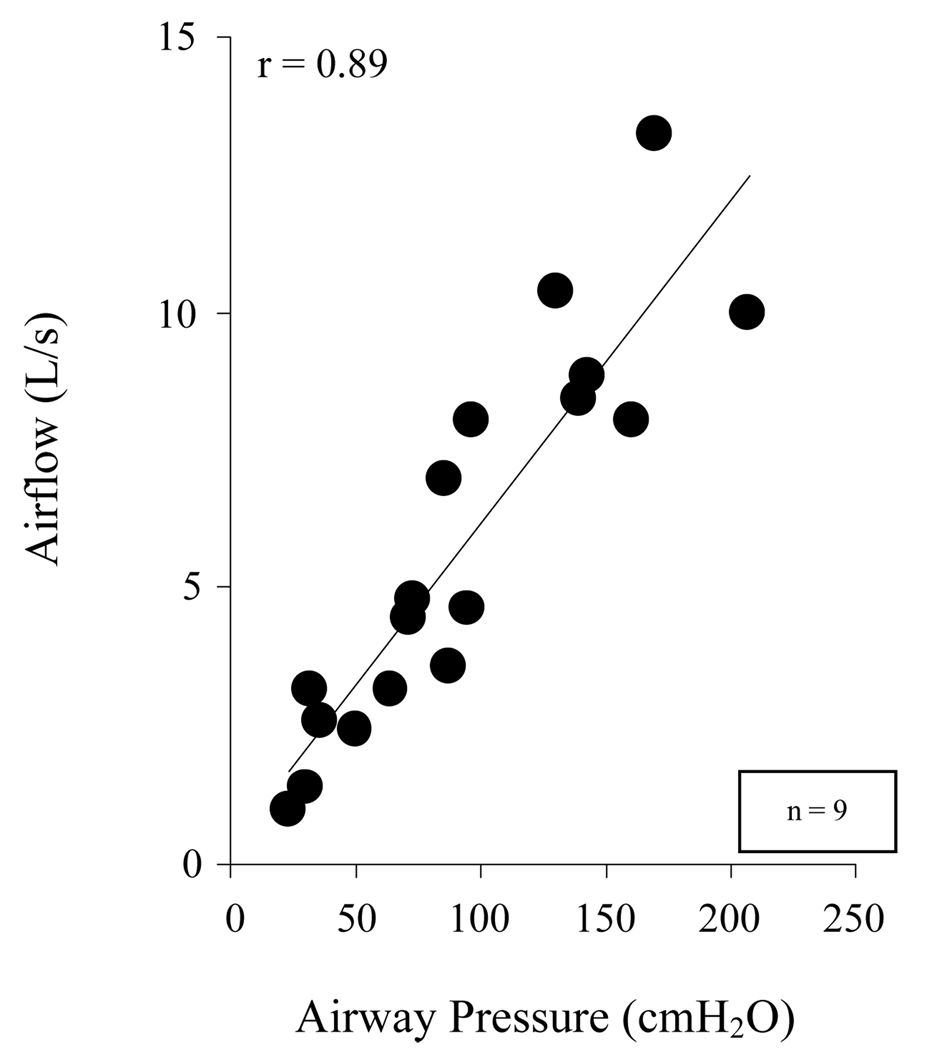
As shown in Figure 7, there was a close relationship between peak airflow and airway pressure generation during supramaximal SCS at TLC and FRC. Airway pressure generation during SCS, therefore, could be used as a reliable indicator of peak expiratory airflow, a parameter which is often used to assess cough efficacy.
Figure 7.
Relationship between airway pressure and peak airflow generation, for each subject at FRC and at TLC. There was a highly significant linear relationship between these parameters (p < 0.01). By this relationship, peak airflow rates could be predicted based upon the magnitude of airway pressure generation.
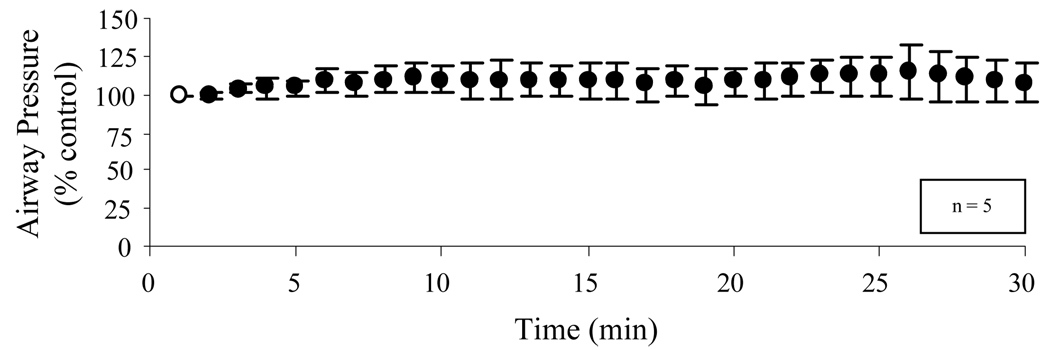
The effects of supramaximal stimulation applied every minute for 30 min is shown in Figure 8. Pressure generation was maintained at or above control values throughout this period indicating the absence of significant system fatigue.
Figure 8.
Mean changes in airway pressure (expressed as a percent maximum) with 2 site SCS applied every 1 min over a 30 min period. There were no significant decrements in airway pressure generation over this time period indicating no evidence of system fatigue during the chronic application of SCS.
Procedurally related complications included mild edema at the receiver site in 5 subjects which generally resolved over several weeks. In one subject, revision of the receiver placement was necessary due to skin folds. In two other subjects, 1 of the 3 leads was not functional. Since only 2 leads were necessary to achieve maximal pressure development, this did not interfere with overall function of the system. No other complications were observed.
Discussion
In a previous case report (25), we presented the results of our initial subject with tetraplegia in whom lower thoracic spinal cord stimulation (SCS) was applied to restore an effective cough mechanism. In this paper, additional clinical experience with this technique is provided. Consistent with our previous report (25), the results of this investigation demonstrate that lower thoracic SCS results in the generation of large airway pressures and peak flow rates, which in several subjects approach values observed during a maximum cough effort in normal individuals (29–32). While mean maximum pressure generation during SCS (137 cmH2O) was less than the maximum expiratory pressure generating capacity of normal individuals (~ 200 cmH2O for males and ~ 150 cmH2O for females) (33), this most likely occurred as a result of the reductions in inspiratory capacity (IC) in our research subject group. The expiratory muscles are positioned at their greatest length and achieve their greatest force generating capacity at TLC; expiratory muscle force generation falls progressively with decreases in lung volume (18). Despite the reduced IC, several subjects achieved maximum airway pressure generation in the normal range. Taken together, this data suggests that lower thoracic SCS results in near maximal activation of the expiratory muscles.
The magnitude of peak flow necessary to maintain an effective cough is not clear. However, in patients with Duchenne muscular dystrophy during periods of upper respiratory tract infection and therefore increased secretions, a peak flow of at least 4.5 L/s was necessary to avoid the development of respiratory failure (34). With the exception of one individual with kyphoscoliosis, each of our research subjects achieved peak flow rates above this value with SCS. In fact, the mean peak flow rate was substantially greater than this value indicating a significant margin of reserve.
Mechanism of Expiratory Muscle Activation
In contrast to prior animal studies (22, 23), the magnitude of pressure development was similar at each of the 3 stimulation sites. In dogs, maximum pressure development occurred with dorsal epidural stimulation at the T9 spinal level (18, 22, 23). At the T11 and L1 spinal levels, pressure generation fell to 72% and 40% of the values obtained at the T9 level, respectively (23). The reasons for these differences are not clear but may relate to anatomic differences between species. For example, the human thorax is much more compressed in the anteroposterior dimension, whereas the quadruped thorax is more compressed in the transverse dimension (35, 36). Conceivably, these shape differences may have altered the mechanical advantage of the expiratory muscles or the transmission of intra-abdominal pressure to the airway.
The mechanism of expiratory muscle activation during lower thoracic SCS has been evaluated extensively in animal studies (18, 22, 23, 37). In dogs, epidural dorsal SCS at the T9 spinal level resulted in direct activation of motor roots in the vicinity of the electrode (~ 2 segments cephalad and ~ 2 segments caudal). In addition, more caudal roots were activated via spinal cord pathways (22, 23). SCS at the T9 level alone, however, resulted in incomplete expiratory muscle activation, as stimulation with a second electrode at the L1 spinal level resulted in significantly greater changes in airway pressure. Stimulation with a third electrode at sites between T9 and T13/L1, however, did not result in any further increases in pressure generation (22, 23). Despite these results in animal studies, a three-electrode system was implanted at the T9, T11 and L1 spinal levels in our clinical trial due to size differences between species. Like the animal studies however, a two-electrode system resulted in similar airway pressures as those generated with a three-electrode system. While initial results obtained from our first subject (25) suggested that the combination of T9 and L1 stimulation resulted in the greatest pressure generation, the group mean data of the present study indicates that any two-electrode combination resulted in similar airway pressure generation. Consistent with the close correlation between airway pressure generation and peak flow rates (Figure 6), similar relationships were observed between sites of stimulation and peak flow rate generation.
Methodological Concerns
The accurate measurement of airway pressure and airflow was highly dependent upon operator technique and subject cooperation. During data collection, several factors often led to an underestimation of actual airflow and pressure generation. First, given the very high airway pressures resulting from SCS, mask leakage was a frequent occurrence. To obtain accurate results therefore, technical assistance was required to hold the mask in place and prevent mask displacement. Manual pressure was also applied to the cheeks during SCS. Mask leakage was usually obvious secondary to associated high pitched sounds; in these instances, SCS was repeated until mask leakage was minimized or eliminated. Second, while subjects were instructed to completely relax and maintain an open glottis during SCS, reflex glottic closure was a frequent occurrence resulting in inaccurately low airway pressure measurement. Large differences in airflow and pressure generation with similar expiratory muscle activation can also occur as a result of differential narrowing of the glottis. With frequent practice maneuvers and training, however, each of the subjects was able to coordinate the maintenance of glottic opening with SCS.
Since maximum spontaneous airway pressure generation was small relative to pressures generated by SCS, there was much less concern about the potential for overestimation of airway pressure generation. Nonetheless, subjects were instructed to completely relax during SCS, and subjects were carefully observed for any signs of effort.
Comparison to other Methods of Expiratory Muscle Activation
Other stimulation techniques have also been proposed to activate the expiratory muscles. These include high-frequency magnetic stimulation (20, 21, 38, 39) and surface abdominal muscle stimulation (17, 19, 40, 41).
Magnetic stimulation of the expiratory muscles requires placement of a stimulating coil over the back at the T10 spinal level (20, 21, 39). Previous investigations have shown that this method results in the generation of large positive airway pressures in normal subjects (21, 38). When applied in subjects with tetraplegia however, airway pressures and flow rates were not significantly different than those generated during spontaneous maximum expiratory efforts (39). Muscle atrophy may have been responsible, in part, for the generation of smaller airway pressures. The major advantages of magnetic stimulation are that it results in only mild discomfort, activates a large portion of the expiratory muscles and can be applied non-invasively (21, 39). Limiting clinical application, however, are several significant disadvantages. The device is bulky, expensive and requires an external power source, which is likely to restrict patient mobility. In addition, the device carries some risk of thermal injury since it generates considerable heat at the stimulating coil. Significant adipose tissue may also interfere with expiratory muscle activation due to the greater distance between the stimulating coil and motor roots.
Several investigations have assessed the potential of surface abdominal muscle stimulation to activate the expiratory muscles (17, 19, 40, 41). In previous studies in subjects with tetraplegia in which electrodes were placed over the anterior abdominal wall, stimulation resulted in only modest increases (~30 cmH2O) in maximum expiratory pressure to ~ 55–60 cmH2O (17, 19, 40). Moreover, peak flow rates with this technique were not significantly different than volitional cough. Importantly, significant abdominal muscle contraction could not be achieved in more than 20% of subjects. In a more recent study in normal subjects, however, surface electrodes (with much larger surface areas) placed over the posterolateral portion of the abdominal wall resulted in twitch pressures comparable to those achieved with magnetic stimulation (41). While also non-invasive, this method also has significant disadvantages. Repeated application of electrodes to the skin surface is likely to be quite tedious and cumbersome and may lead to skin irritation and breakdown, a common problem in patients with SCI. Moreover, the presence of adipose tissue in obese patients may prevent successful application due to the high electrical resistance of fatty tissue.
The SCS technique presented in the present study is highly portable and does not require the repeated application and removal of electrodes. Moreover, the degree of expiratory muscle activation should not be affected by the presence of significant adipose tissue. While this method does require an invasive procedure, the surgical technique for electrode placement is standard; SCS has been in clinical use for over 35 years in the treatment of chronic back pain and spasticity (42–45). Most complications of the procedure relate to equipment failure, which can range as high as 20–25%. In this present study, 2 of 27 leads were non-functional. However, this did not interfere with the function of the system. The incidence of operative complications such as infection and bleeding, however, are quite low (42–45). While deep infection is rare (42), superficial infections have been observed in 4–6% of patients (42–45). No infections were observed in the present study. Future development of this technique should include the evaluation of wire electrodes which can be implanted much less invasively and may also achieve adequate expiratory muscle activation.
Conclusion
In conclusion, lower thoracic SCS results in near maximal activation of the expiratory muscles with the consequent generation of high airway pressures and peak airflow rates, characteristic of a normal cough. Restoration of an effective cough by this method has the potential to facilitate removal of secretions, reduce the incidence of respiratory tract infections and atelectasis and associated morbidity and mortality in subjects with SCI.
Acknowledgement
The authors gratefully acknowledge the technical assistance in data analysis of Tomasz Kowalski.
Support: This project was supported by the National Institute of Neurological Disorders and Stroke (R01 NS049516) and by the Clinical Research Unit at MetroHealth Medical Center, supported by Case Western Reserve University School of Medicines CTSA Award, UL1-RR024989, Pamela Davis, M.D. PI, from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Abstracts/Presentations:
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce an effective cough in spinal cord injured patients. Presented at the Congress of Neurological Surgeons Annual Meeting; Chicago, IL, October 2006.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation (SCS) to restore cough in patients with spinal cord injury. Presented at the American Spinal Injury Association International Conference; Tampa, FL, May – June 2007. J Spinal Cord Med. 30:176, 2007.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation (SCS) to restore cough in patients with spinal cord injury. Presented at the International Spinal Cord Society Meeting; Reykjavik, Iceland, June 2007.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey G. Muscle reconditioning for cough production in spinal cord injured patients. Presented at the American Paraplegia Society Annual Conference; Orlando, FL, August 2007. J Spinal Cord Med. 30: 405, 2007.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation (SCS) to restore cough in patients with spinal cord injury. Presented at the American Academy of Physical Medicine and Rehabilitation Annual Assembly; Boston, MA, September 2007.
DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Nemunaitis GA. Cough generation via spinal cord stimulation (SCS) in spinal cord injury. Am J Respir Crit Care Med 2008;177:A272. May 16–21, 2008.
Romaniuk JR, Hromyak DR, Geertman RT, Kowalski KE, DiMarco AF. Patient satisfaction following restoration of a physiologic cough in spinal cord injury. J Spinal Cord Med (in press).
Kowalski KE, Geertman RT, Hromyak DR, DiMarco AF. Activation of expiratory muscles for cough production in tetraplegics. J Spinal Cord Med (in press).
Disclosure: We certify that we have affiliations with or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants and patents received or pending, royalties) with an organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript AND all such affiliations and involvements are disclosed on the title page of the manuscript.
Explanation of Disclosure: Dr. DiMarco is a Founder of and has a significant financial interest in Synapse BioMedical, Inc, a manufacturer of diaphragm pacing systems.
Clinical Trial Registration Number: NCT00116337
Suppliers
Freehand Epimysial Electrode; NeuroControl Corp., Valley View, OH
Finetech Medical Ltd., Welwyn Garden City, Herfordshire, UK
Validyne MP45; Validyne Co., Northridge, CA
Fleisch No. 1; Fleisch, Lausanne, Switzerland
DASH8; AstroMed Inc., West Warwick, RI
Nellcor N-200; Nellcor, Boulder, CO
References
- 1.DeTroyer A, Estenne M, Heilporn A. Mechanism of active expiration in tetraplegic subjects. N Engl J Med. 1986;314:740–744. doi: 10.1056/NEJM198603203141203. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, DeTroyer A. Cough in tetraplegic subjects: an active process. Ann Intern Med. 1990;112:22–28. doi: 10.7326/0003-4819-112-1-22. [DOI] [PubMed] [Google Scholar]
- 3.Kelly BJ, Luce JM. The diagnosis and management of neuromuscular diseases causing respiratory failure. Chest. 1991;99:1485–1494. doi: 10.1378/chest.99.6.1485. [DOI] [PubMed] [Google Scholar]
- 4.McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia: recognition, prevention and treatment. JAMA. 1980;243:528–531. [PubMed] [Google Scholar]
- 5.Carter RE. Respiratory aspects of spinal cord injury management. Paraplegia. 1987;25:262–266. doi: 10.1038/sc.1987.48. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75:270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 7.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–870. [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30:319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homnick DN. Mechanical insufflation-exsufflation for airway mucus clearance. Respir Care. 2007;52:1296–1307. [PubMed] [Google Scholar]
- 10.Sivasothy P, Brown L, Smith IE, Shneerson JM. Effect of manually assisted cough and mechanical insufflation on cough flow of normal subjects, patients with chronic obstructive pulmonary disease (COPD), and patients with respiratory muscle weakness. Thorax. 2001;56:438–444. doi: 10.1136/thorax.56.6.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J. 2003;21:502–508. doi: 10.1183/09031936.03.00048102. [DOI] [PubMed] [Google Scholar]
- 12.Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993;104:1553–1562. doi: 10.1378/chest.104.5.1553. [DOI] [PubMed] [Google Scholar]
- 13.Kiwerski JE. Factors contributing to the increased threat of life following spinal cord injury. Paraplegia. 1993;31:793–799. doi: 10.1038/sc.1993.122. [DOI] [PubMed] [Google Scholar]
- 14.Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F. Survival and cause of death after traumatic spinal cord injury: a long-term epidemiological survey from Denmark. Spinal Cord. 1997;35:76–85. doi: 10.1038/sj.sc.3100351. [DOI] [PubMed] [Google Scholar]
- 15.Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- 16.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 17.Jaeger RJ, Langbein EW, Kralj AR. Augmenting cough by FES in tetraplegia: a comparison of results at three clinical centers. Basic Appl Myol. 1994;4:195–200. [Google Scholar]
- 18.DiMarco AF, Romaniuk JR, Supinski GS. Electrical activation of the expiratory muscles to restore cough. Am J Respir Crit Care Med. 1995;151:1466–1471. doi: 10.1164/ajrccm.151.5.7735601. [DOI] [PubMed] [Google Scholar]
- 19.Jaeger RJ, Turba RM, Yarkony GM, Roth EJ. Cough in spinal cord injured patients: comparison of three methods to produce cough. Arch Phys Med Rehabil. 1993;74:1358–1361. doi: 10.1016/0003-9993(93)90093-p. [DOI] [PubMed] [Google Scholar]
- 20.Lin VWH, Hsieh C, Hsiao IN, Canfield J. Functional magnetic stimulation of expiratory muscles: a noninvasive and new method for restoring cough. J Appl Physiol. 1998;84:1144–1150. doi: 10.1152/jappl.1998.84.4.1144. [DOI] [PubMed] [Google Scholar]
- 21.Kyroussis D, Polkey MI, Mills GH, Hughes PD, Moxham J, Green M. Stimulation of cough in man by magnetic stimulation of the thoracic nerve roots. Am J Respir Crit Care Med. 1997;156:1696–1699. doi: 10.1164/ajrccm.156.5.9702008. [DOI] [PubMed] [Google Scholar]
- 22.DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Pattern of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 1999;86:1881–1889. doi: 10.1152/jappl.1999.86.6.1881. [DOI] [PubMed] [Google Scholar]
- 23.DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 2002;92:2341–2346. doi: 10.1152/japplphysiol.01231.2001. [DOI] [PubMed] [Google Scholar]
- 24.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH, Nemunaitis GA. Lower Thoracic Spinal Cord Stimulation to Restore Cough in Patients with Spinal Cord Injury: Results of an NIH Sponsored Clinical Trial; Part II: Clinical Outcomes. Submitted to Arch Phys Med Rehabil. 2008 doi: 10.1016/j.apmr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Spinal cord stimulation: a new method to produce an effective cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006;173:1386–1389. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMarco AF, Kowalski KE. Effects of chronic electrical stimulation on paralyzed expiratory muscles. J Appl Phisol. 2008;104:1634–1640. doi: 10.1152/japplphysiol.01321.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson AS, Stone HE, Roessmann U, Burke M, Tisdale E, Mortimer JT. Muscle plasticity: comparison of a 30-Hz burst with 10-Hz continuous stimulation. J Appl Physiol. 1989;66:1143–1151. doi: 10.1152/jappl.1989.66.3.1143. [DOI] [PubMed] [Google Scholar]
- 28.Pette D, Vrbova G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. 1999. [DOI] [PubMed] [Google Scholar]
- 29.Loudon RG, Shaw GB. Mechanics of cough in normal subjects and in patients with obstructive respiratory disease. Am Rev Respir Dis. 1967;96:666–677. doi: 10.1164/arrd.1967.96.4.666. [DOI] [PubMed] [Google Scholar]
- 30.Langlands J. The dynamics of cough in health and in chronic bronchitis. Thorax. 1967;22:88–96. doi: 10.1136/thx.22.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora NS, Gal TJ. Cough dynamics during progressive expiratory muscle weakness in healthy curarized subjects. J Appl Physiol. 1981;51:494–498. doi: 10.1152/jappl.1981.51.2.494. [DOI] [PubMed] [Google Scholar]
- 32.Siebens AA, Kirby NA, Poulos DA. Cough following transection of spinal cord at C-6. Arch Phys Med Rehabil. 1964;45:1–8. [PubMed] [Google Scholar]
- 33.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 34.Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997;112:1024–1028. doi: 10.1378/chest.112.4.1024. [DOI] [PubMed] [Google Scholar]
- 35.Woodburne RT. Essentials of human anatomy. New York: Oxford University Press; 1969. [Google Scholar]
- 36.Miller ME, Chrisensen GC, Eveans E. Anatomy of the dog. Philadelphia: WB Saunders; 1964. [Google Scholar]
- 37.DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Mechanical contribution of expiratory muscles to pressure generation during spinal cord stimulation. J Appl Physiol. 1999;87:1433–1439. doi: 10.1152/jappl.1999.87.4.1433. [DOI] [PubMed] [Google Scholar]
- 38.Polkey MI, Luo Y, Guleria R, Hamnegård CH, Green M, Moxham J. Functional magnetic stimulation of the abdominal muscles in humans. Am J Respir Crit Care Med. 1999;160:513–522. doi: 10.1164/ajrccm.160.2.9808067. [DOI] [PubMed] [Google Scholar]
- 39.Lin VWH, Singh H, Chitkara RK, Perkash I. Functional magnetic stimulation for restoring cough in patients with tetraplegia. Arch Phys Med Rehabil. 1998;79:517–522. doi: 10.1016/s0003-9993(98)90065-x. [DOI] [PubMed] [Google Scholar]
- 40.Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest. 1993;103:166–169. doi: 10.1378/chest.103.1.166. [DOI] [PubMed] [Google Scholar]
- 41.Lim J, Gorman RB, Saboisky JP, Gandevia SC, Butler JE. Optimal electrode placement for noninvasive electrical stimulation of human abdominal muscles. J Appl Physiol. 2007;102:1612–1617. doi: 10.1152/japplphysiol.00865.2006. [DOI] [PubMed] [Google Scholar]
- 42.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137–147. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine. 2004;30:152–160. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 45.Kumar K, Buchser E, Linderoth B, Meglio M, Van Buyten JP. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation. 2007;10:24–33. doi: 10.1111/j.1525-1403.2007.00084.x. [DOI] [PubMed] [Google Scholar]