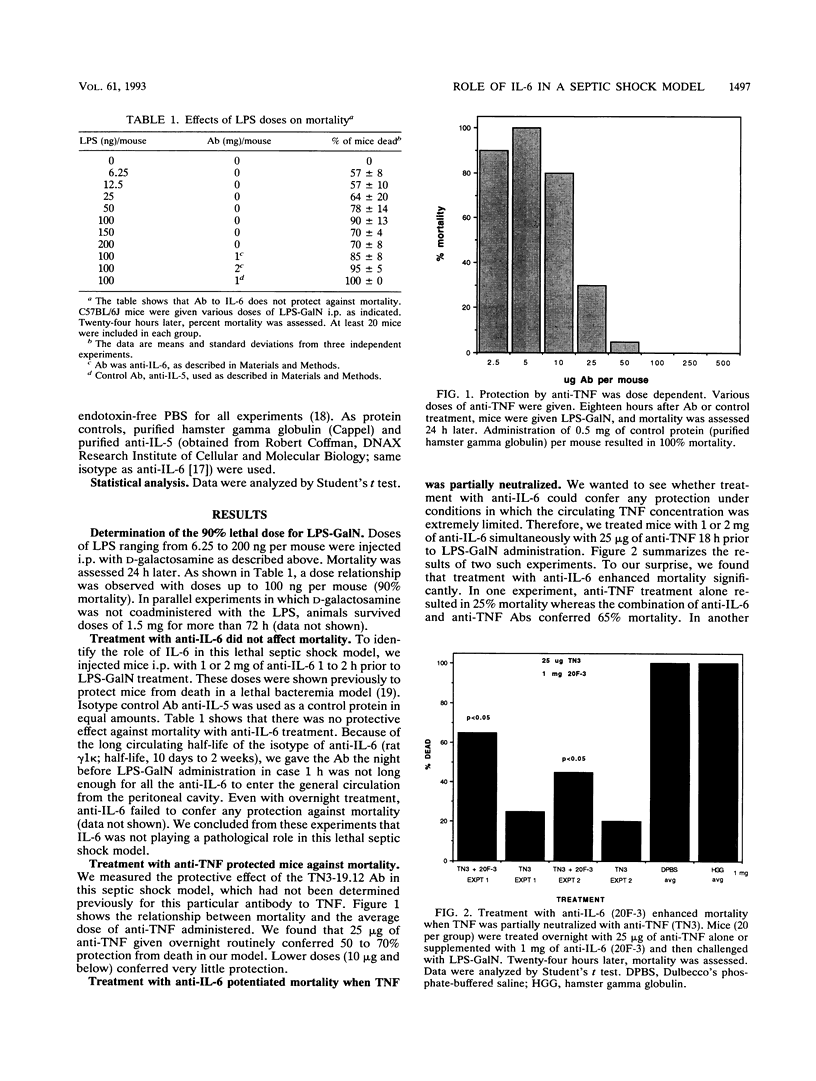
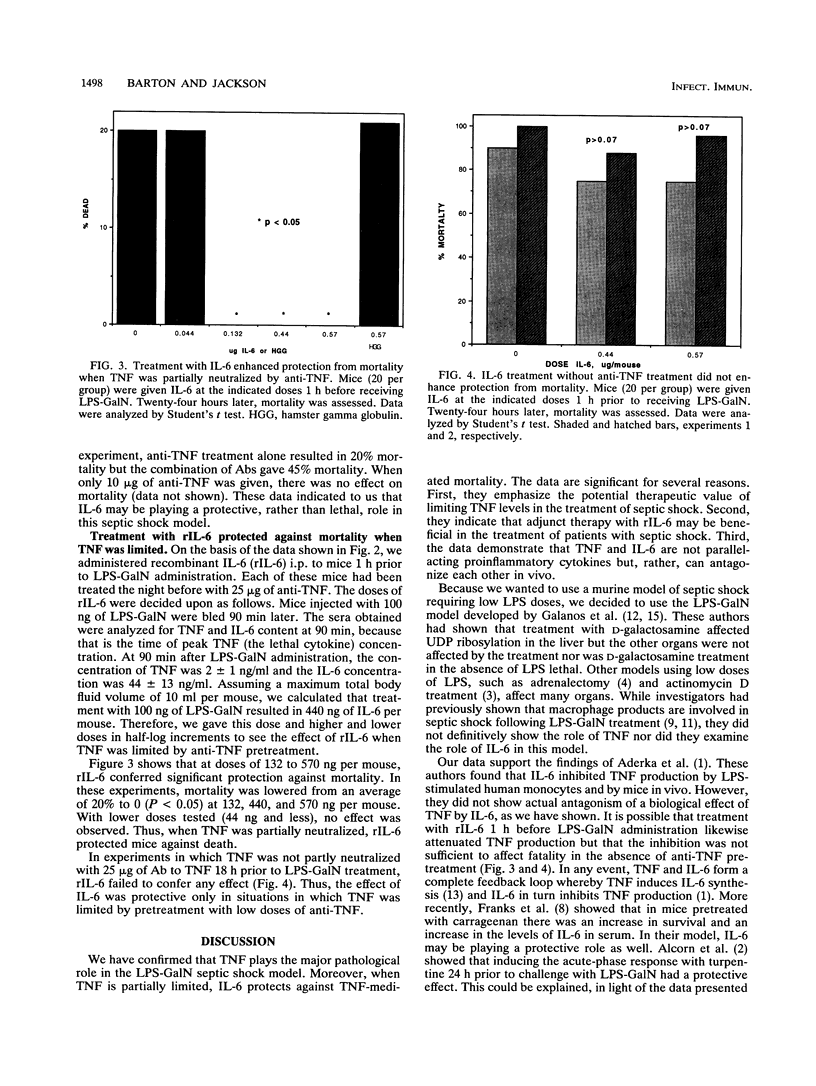
Abstract
C57BL/6J mice given low doses of lipopolysaccharide (LPS) (100 ng per mouse) plus D-galactosamine (8 mg per mouse) die within 24 h following LPS administration. We used this septic shock model to confirm the role of tumor necrosis factor in mortality using a monoclonal antibody to tumor necrosis factor to prevent lethality. Furthermore, we demonstrated that interleukin 6, rather than playing a lethal role, protected mice against death in this septic shock model. Antibody to interleukin 6 did not affect the fatal outcome in this low-LPS-dose model. However, pretreatment with antibody to tumor necrosis factor did protect the mice against death, in a dose-dependent manner. Moreover, mortality was enhanced by pretreatment with antibody to interleukin 6 when tumor necrosis factor was partly limited by anti-tumor necrosis factor treatment. Mortality was significantly reduced by pretreatment with both recombinant interleukin 6 and low doses of antibody to tumor necrosis factor; in the absence of the low dose of antibody to tumor necrosis factor, interleukin 6 alone did not confer any protection. These data demonstrate in vivo antagonistic activities of tumor necrosis factor and interleukin 6 and show that interleukin 6 can play a protective role against death from septic shock.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Alcorn J. M., Fierer J., Chojkier M. The acute-phase response protects mice from D-galactosamine sensitization to endotoxin and tumor necrosis factor-alpha. Hepatology. 1992 Jan;15(1):122–129. doi: 10.1002/hep.1840150121. [DOI] [PubMed] [Google Scholar]
- BERRY L. J., SMYTHE D. S. EFFECTS OF BACTERIAL ENDOTOXINS ON METABOLISM. VII. ENZYME INDUCTION AND CORTISONE PROTECTION. J Exp Med. 1964 Nov 1;120:721–732. doi: 10.1084/jem.120.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fong Y., Tracey K. J., Moldawer L. L., Hesse D. G., Manogue K. B., Kenney J. S., Lee A. T., Kuo G. C., Allison A. C., Lowry S. F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med. 1989 Nov 1;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks A. K., Kujawa K. I., Yaffe L. J. Experimental elimination of tumor necrosis factor in low-dose endotoxin models has variable effects on survival. Infect Immun. 1991 Aug;59(8):2609–2614. doi: 10.1128/iai.59.8.2609-2614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Koj A., Dubin A. Inhibition of the liver and plasma protein acute-phase response in mice by D-galactosamine. Br J Exp Pathol. 1976 Dec;57(6):733–741. [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]


