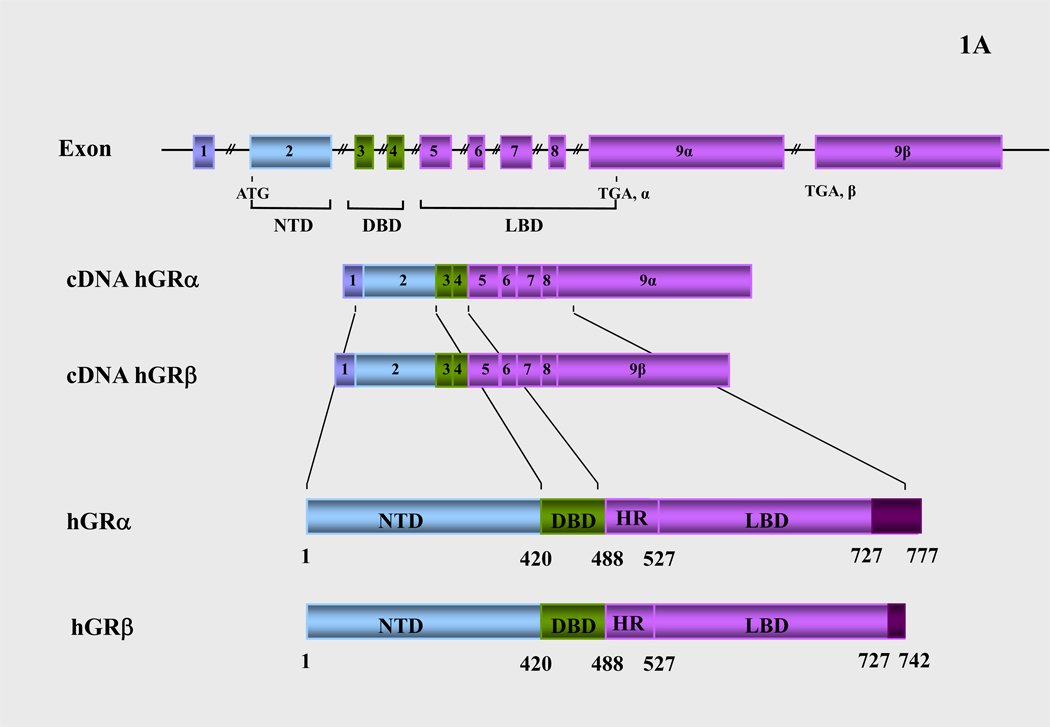
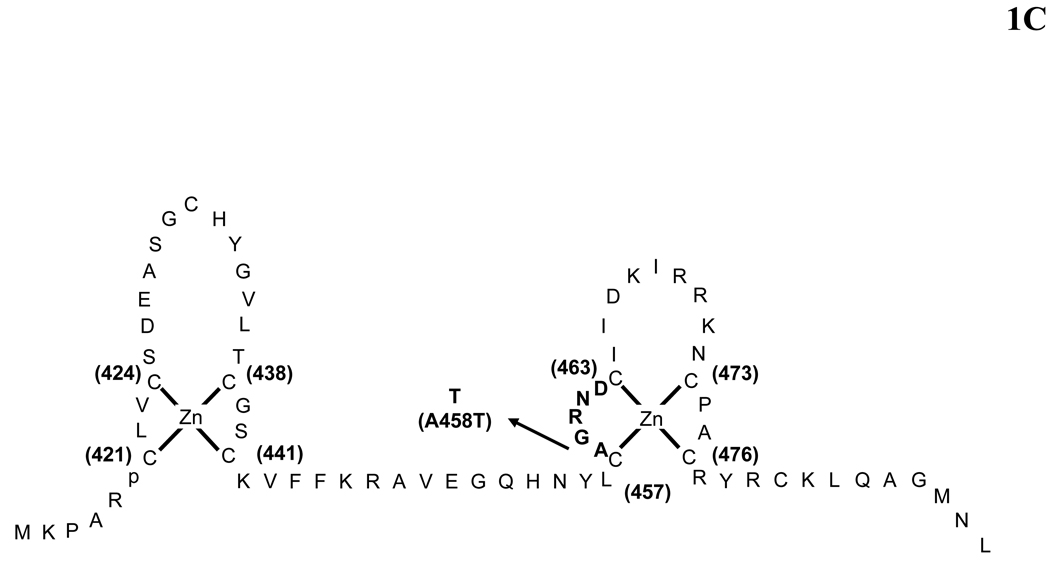
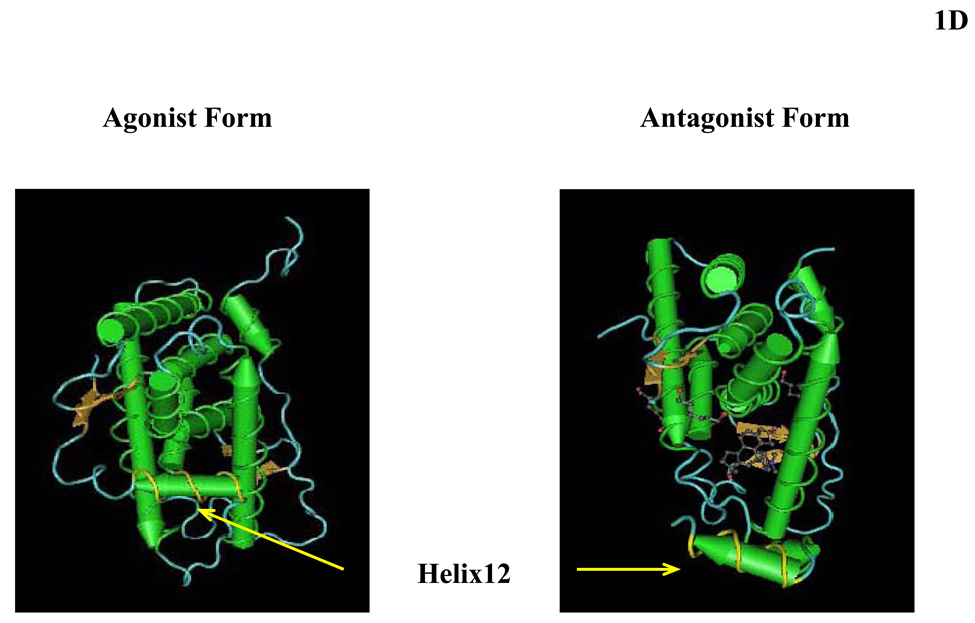
Figure 1.
(A) Schematic representation of the structure of the human glucocorticoid receptor (hGR) gene. Alternative splicing of the primary transcript gives rise to the two mRNA and protein isoforms, hGRα and hGRβ (B) Functional domains of the hGRα. The functional domains and subdomains are indicated beneath the linearized protein structures. AF, activation function; DBD, DNA-binding domain; LBD, ligand-binding domain; NLS, nuclear localization signal. (C) Enlargement of part of the DBD showing the amino acid sequence (single letter codes) of the two zinc fingers and the dimerization loop (in bold). The A to T mutation at position 458 that could produce a dimerization defective receptor is shown. (D) Crystal structure of the ligand-binding domain (LBD) of the human glucocorticoid receptor-α (hGRα). Stereotactic conformation of the agonist (left) and antagonist (right) form of the LBD of hGRα. The yellow arrows indicate the position of Helix 12, which is critical for the formation of AF-2 surface that allows interaction with activators (Adapted from References 78–80).