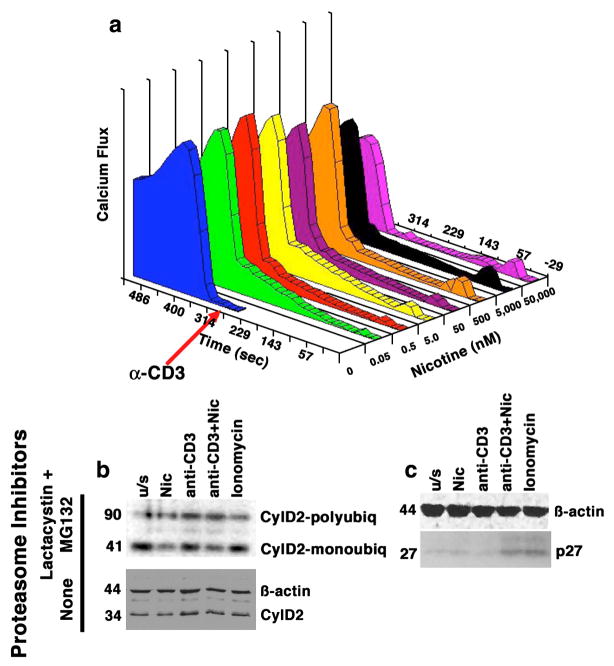
Figure 1. Dose-dependent calcium mobilization in human T cells induced by nicotine has functional consequences.
Panel a shows Jurkat cells treated with nicotine at the indicated concentrations for 5 min prior to the addition of anti-CD3 (10 ng/ml). Alterations in Cai2+ were measured using a MoFlo flow cytometer. Data on the X-axis represent nicotine dose (nM), on the Y-axis, time (sec) and on the Z-axis (Calcium flux) they are expressed as the product of excitation X proportion of responding cells. Panel b shows Jurkat cells cultured as indicated in the presence or absence of proteasome inhibitors Lactacystin and MG132. Cyclin D2 complexes were immunoprecipitated using anti-cyclin D2 antibody and immunoblotted with an anti-ubiquitin antibody (top). Monoubiquitinated Cyclin D2 complexes migrate with an apparent MW of 41 kDa; polyubiquitinated complexes migrate with an apparent MW of ~90 kDa (compared to the 34 kDa native protein). The ratio of polyubiquitinated to monoubiquitinated Cyclin D2 was 2.5-fold and 2-fold greater, respectively, in cells treated with nicotine or with anti-CD3 than in unstimulated cells. The effects of nicotine and anti-CD3 were additive, with the ratio increasing to 3.2-fold over untreated cells when both compounds were used together. The lower immunoblot shows levels of Cyclin D2 in whole cell lysates from cells stimulated in an identical manner without proteasome inhibitors. Panel c shows the levels of the p27 CDK inhibitor in whole cell lysates from primary T cells stimulated in an identical manner in the absence of proteasome inhibitors. The steady state levels of p27 were significantly different (5-fold greater) in cells treated with nicotine and anti-CD3 together, or with ionomycin than in untreated cells. Ionomycin was included in the experiments shown in panel b and panel c to control for non-specific effects of calcium mobilization, and ϐ-actin immunoblots are included as loading controls.