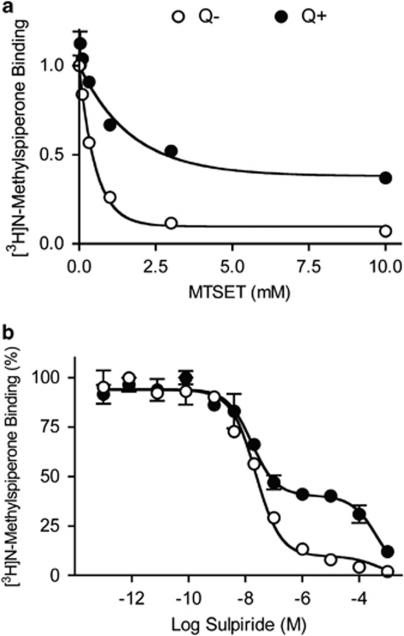
Figure 3.
Effect of MTSET and competition with sulpiride on [3H]NMSP binding. Binding assays were carried out in intact cells using [3H]NMSP and quinpirole to induce internalization. (a) Surface receptors were inactivated with increasing concentrations of MTSET. Under control conditions (○, Q−), MTSET inactivated 90% of [3H]NMSP binding. After quinpirole treatment (•, Q+), MTSET inactivated only about 55% of [3H]NMSP binding, indicating that about 45% of D2 receptor underwent internalization. (b) Surface receptors were blocked with the relatively membrane-impermeant antagonist sulpiride. Under control conditions (○, Q−), sulpiride competition binding showed a two-site [3H]NMSP binding curve; the majority of D2 receptors (90%) were accessible to sulpiride and thus subject to competition at high affinity (Ki(s)=9.1±1.0 nM), whereas only a small minority (10%), presumably internalized, were relatively inaccessible and hence subject to competition at low affinity (Ki(i)=142.5±26.8 μM). Following quinpirole treatment (•, Q+), the high-affinity [3H]NMSP binding was reduced to 55%, whereas the low-affinity binding increased to about 45%, consistent with robust D2 receptor internalization.