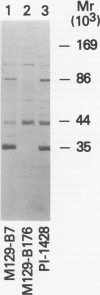
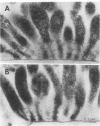
Abstract
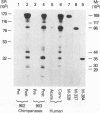
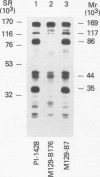
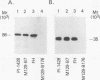
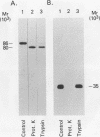
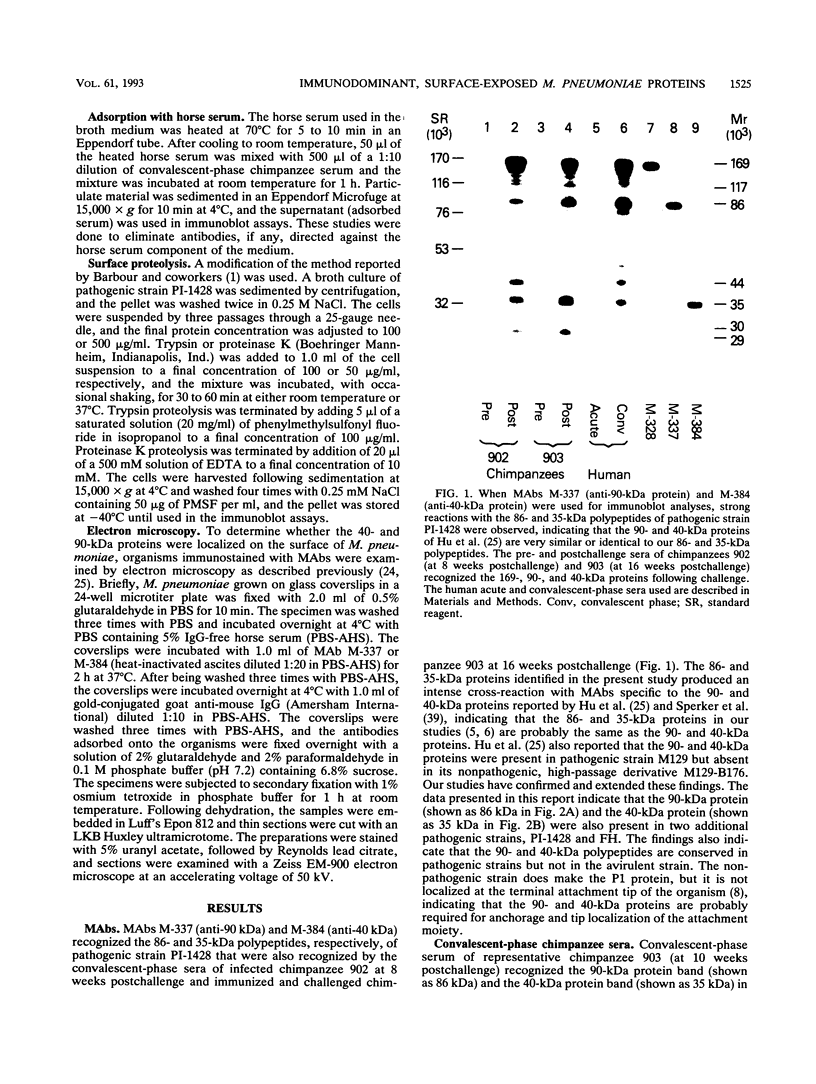
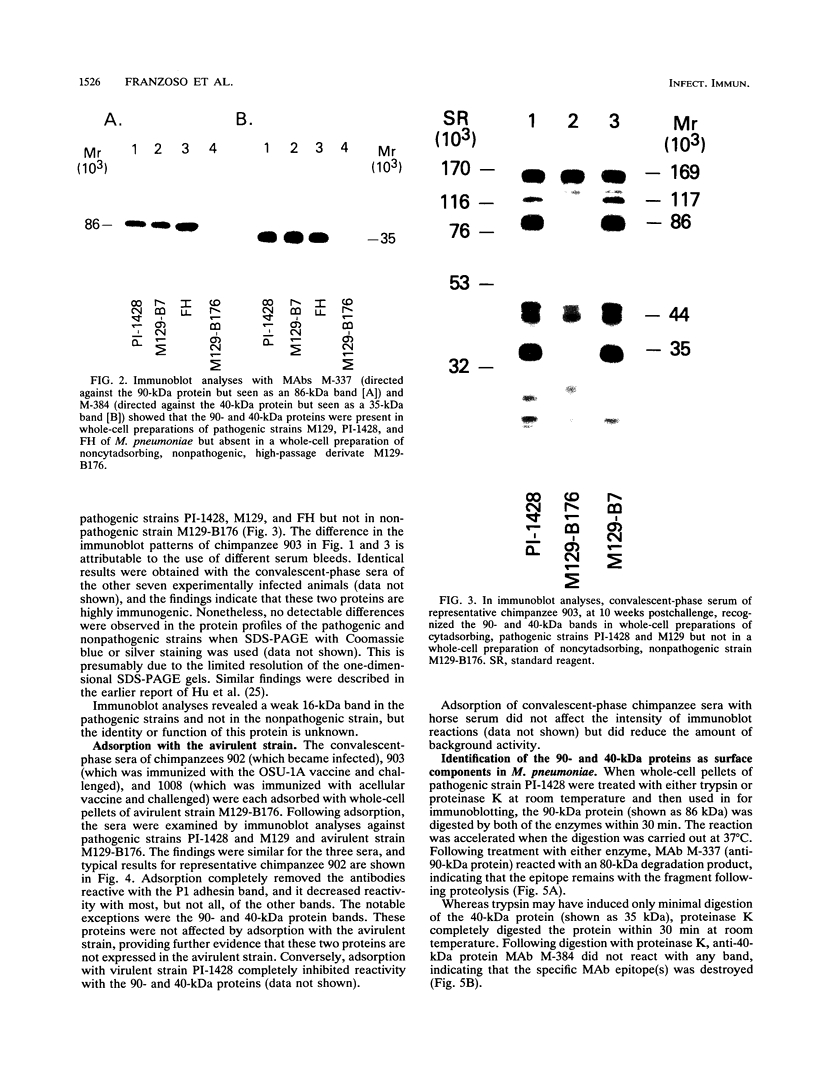
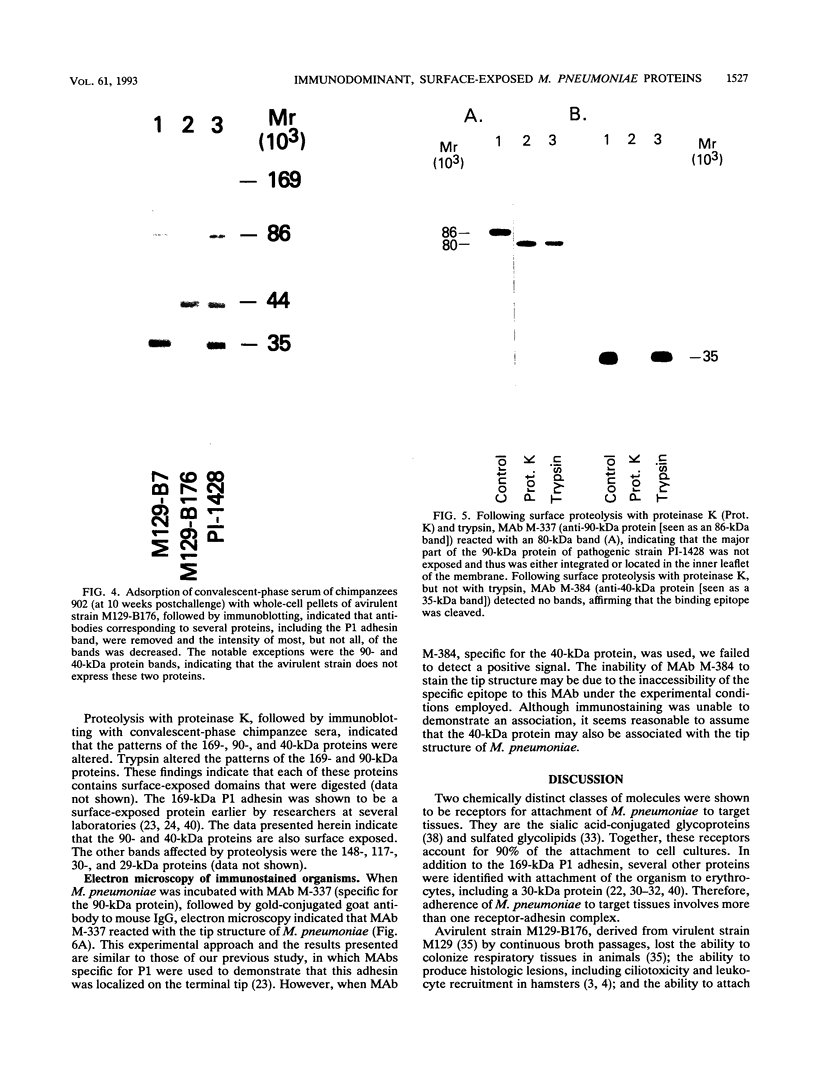
Immunoblot analysis of convalescent-phase sera of experimentally infected chimpanzees or monoclonal antibodies (MAbs) specific to the 90- and 40-kDa proteins of Mycoplasma pneumoniae indicated that both proteins were present in cytadsorbing, pathogenic strains PI-1428, M129, and FH but absent in noncytadsorbing, nonpathogenic strain M129-B176. Adsorption of convalescent-phase chimpanzee sera with virulent strain PI-1428 removed reactivity, whereas adsorption with avirulent strain M129-B176 did not remove reactivity to these two proteins. By using proteolysis and specific MAbs, we demonstrated that the 90- and 40-kDa proteins were surface exposed. Immunoelectron microscopy employing specific MAbs showed that the 90-kDa protein is localized on the terminal tip attachment apparatus. However, the MAb specific for the 40-kDa protein failed to indicate a similar localization. Nevertheless, these data, taken together, indicate that the immunodominant 90- and 40-kDa proteins are surface exposed, are localized on the terminal tip apparatus, and might be involved in the attachment mechanism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Razin S. Hamster challenge potency assay for evaluation of Mycoplasma pneumoniae vaccines. Infect Immun. 1988 Sep;56(9):2450–2457. doi: 10.1128/iai.56.9.2450-2457.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Grabowski M. W., Snoy P. J., Chandler D. K. Superiority of the chimpanzee animal model to study the pathogenicity of known Mycoplasma pneumoniae and reputed mycoplasma pathogens. Isr J Med Sci. 1987 Jun;23(6):556–560. [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Morrison-Plummer J., Drouillard D., Puleo-Scheppke B., Tryon V. V., Holt S. C. Identification of a 32-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Isr J Med Sci. 1987 May;23(5):474–479. [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Razin S., Stephens E. B., Harasawa R., Barile M. F. Genomic and phenotypic analyses of Mycoplasma pneumoniae strains. Infect Immun. 1982 Nov;38(2):604–609. doi: 10.1128/iai.38.2.604-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Bredt W., Kahane I. Influence of cell shape and surface charge on attachment of Mycoplasma pneumoniae to glass surfaces. J Bacteriol. 1983 Jan;153(1):1–5. doi: 10.1128/jb.153.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D., Davies H. A., Dourmashkin R. R. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: characterization of the in vitro model. Infect Immun. 1979 Jul;25(1):446–454. doi: 10.1128/iai.25.1.446-454.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenecker B., Jacobs E. Topological mapping of the P1-adhesin of Mycoplasma pneumoniae with adherence-inhibiting monoclonal antibodies. J Gen Microbiol. 1990 Mar;136(3):471–476. doi: 10.1099/00221287-136-3-471. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Clyde W. A., Jr Serological comparison of virulent and avirulent Mycoplasma pneumoniae by monoclonal antibodies. Isr J Med Sci. 1984 Sep;20(9):870–873. [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Collier A. M., Clyde W. A., Jr Demonstration of antibodies to Mycoplasma pneumoniae attachment protein in human sera and respiratory secretions. Infect Immun. 1983 Jul;41(1):437–439. doi: 10.1128/iai.41.1.437-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Loechel S., Hu P. C. Analysis of the nucleotide sequence of the P1 operon of Mycoplasma pneumoniae. Gene. 1988 Dec 15;73(1):175–183. doi: 10.1016/0378-1119(88)90323-x. [DOI] [PubMed] [Google Scholar]
- Kahane I., Tucker S., Leith D. K., Morrison-Plummer J., Baseman J. B. Detection of the major adhesin P1 in triton shells of virulent Mycoplasma pneumoniae. Infect Immun. 1985 Dec;50(3):944–946. doi: 10.1128/iai.50.3.944-946.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Baseman J. B. Reacquisition of specific proteins confers virulence in Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):830–836. doi: 10.1128/iai.39.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layh-Schmitt G., Herrmann R. Localization and biochemical characterization of the ORF6 gene product of the Mycoplasma pneumoniae P1 operon. Infect Immun. 1992 Jul;60(7):2906–2913. doi: 10.1128/iai.60.7.2906-2913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. D., Olson L. D., Barile M. F., Ginsburg V., Krivan H. C. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989 Jun 5;264(16):9289–9293. [PubMed] [Google Scholar]
- Sperker B., Hu P., Herrmann R. Identification of gene products of the P1 operon of Mycoplasma pneumoniae. Mol Microbiol. 1991 Feb;5(2):299–306. doi: 10.1111/j.1365-2958.1991.tb02110.x. [DOI] [PubMed] [Google Scholar]
- Stevens M. K., Krause D. C. Localization of the Mycoplasma pneumoniae cytadherence-accessory proteins HMW1 and HMW4 in the cytoskeletonlike Triton shell. J Bacteriol. 1991 Feb;173(3):1041–1050. doi: 10.1128/jb.173.3.1041-1050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. H., Collier A. M. Ultrastructural study of Mycoplasma pneumoniae in organ culture. J Bacteriol. 1976 Jan;125(1):332–339. doi: 10.1128/jb.125.1.332-339.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayoshi M., Sasaki T., Yoshioka M. Relationship between an 85 kDa protein and the protective effects of Mycoplasma pneumoniae. Microbiol Immunol. 1992;36(5):455–464. doi: 10.1111/j.1348-0421.1992.tb02044.x. [DOI] [PubMed] [Google Scholar]