Table 3.
Three-Component Coupling of Linchpin 11 with Various Nucleophiles and CCR with Phenyl Iodide
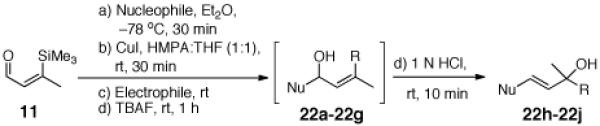 | |||
|---|---|---|---|
| entry | nucleophile | product | |
| 1 |  |
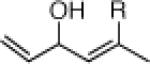 22a: (62%)b,c 22b: (45%)b,d |
22h: (64%)a |
| 2 |  |
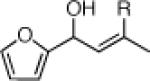 22c: (65%)b,c 22d: (50%)b,d |
 22i: (56%)a |
| 3 | 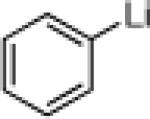 |
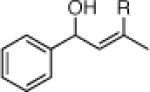 22e: (66%)b,c 22f: (51%)b,d |
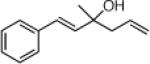 22j: (61%)a |
| 4 | 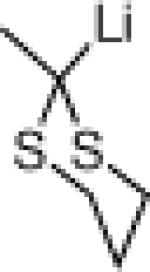 |
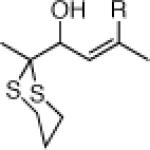 22g: (58%)a,c,e |
NR |
Conditions:
1 N HCl, rt, 10 min.
TBAF, rt, 1 h.
R = Allyl, Allyl bromide, rt, 2 h.
R = Ph, 3 mol % Pd(PPh3)4, Phenyl iodide THF, rt, 6 h.
No cross-coupling product was obtained due to catalyst poisoning by dithiane.
