Fig. 2.
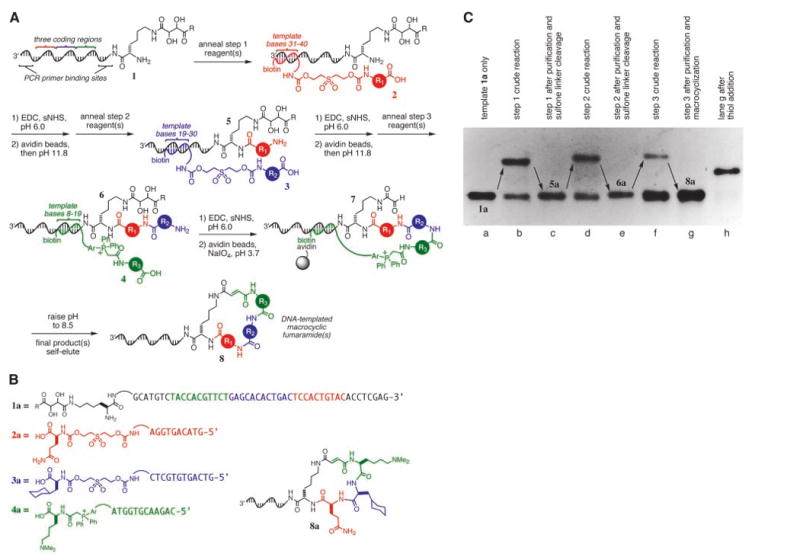
(A) DNA-templated macrocycle library synthesis scheme. R is NHCH3 where R is NHCH3 or tryptamine; Ar, –(p-C6H4)–. The macrocyclization reaction is confirmed to give predominantly trans alkene stereochemistry for one library member (fig. S4) (13) but may give other outcomes for different macrocyclic structures. (B) Template and reagents used in the DNA-templated synthesis of 8a. (C) Denaturing PAGE of each step in the DNA-templated synthesis of 8a. Lane h is the product of a DNA-templated thiol addition to the product shown in lane g, confirming the formation of the fumaramide group during macrocyclization.
