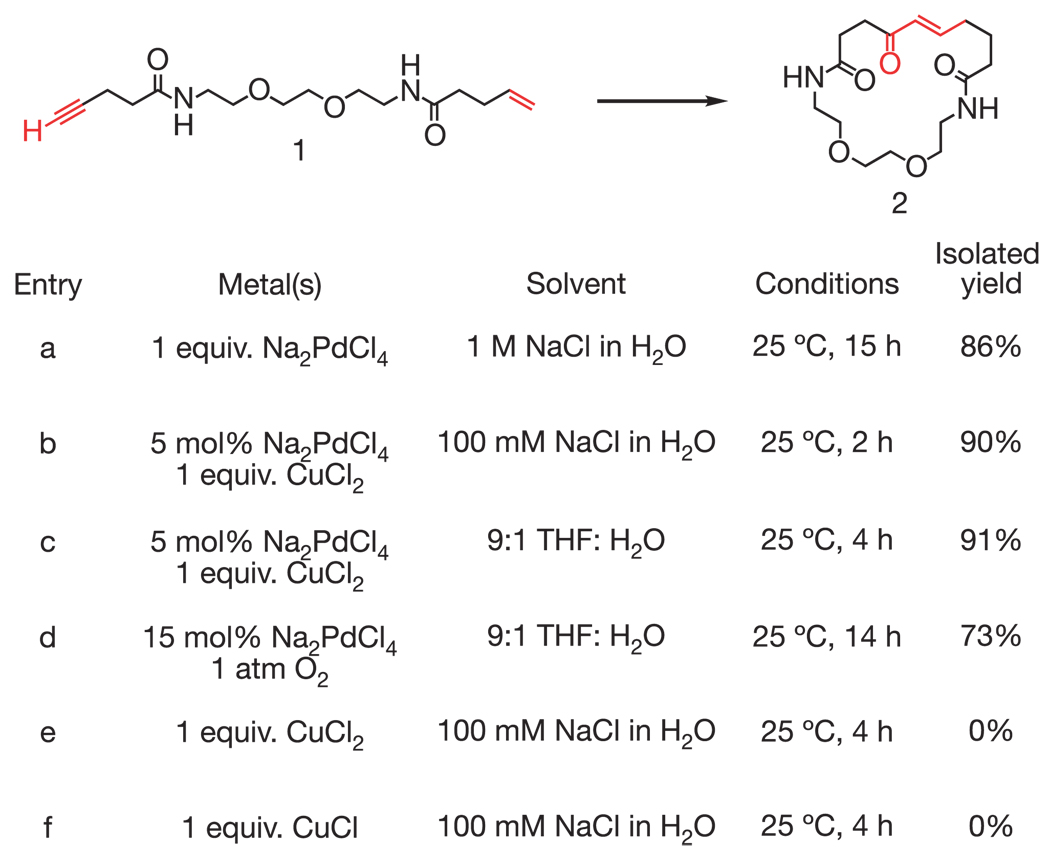
Figure 4.
Characterization of a new alkyne–alkene macrocyclization reaction in a non-DNA-templated format. The macrocyclic enone product (2) was characterized by 1H-NMR, 13C-NMR, COSY, UV–visible spectrometry and high-resolution mass electrospray (Supplementary Information). We speculate that product formation proceeds through the following sequence: soft deprotonation of the alkyne to form a Pd(II)–alkynyl intermediate; insertion of the alkene into the Pd-alkyne bond; β-hydride elimination to form a conjugated enyne; Pd(II)-catalysed hydration of the alkyne to form an enol π-allyl Pd complex; enol tautomerization and π-allyl Pd protonation to generate the trans-enone.