Fig. 4.
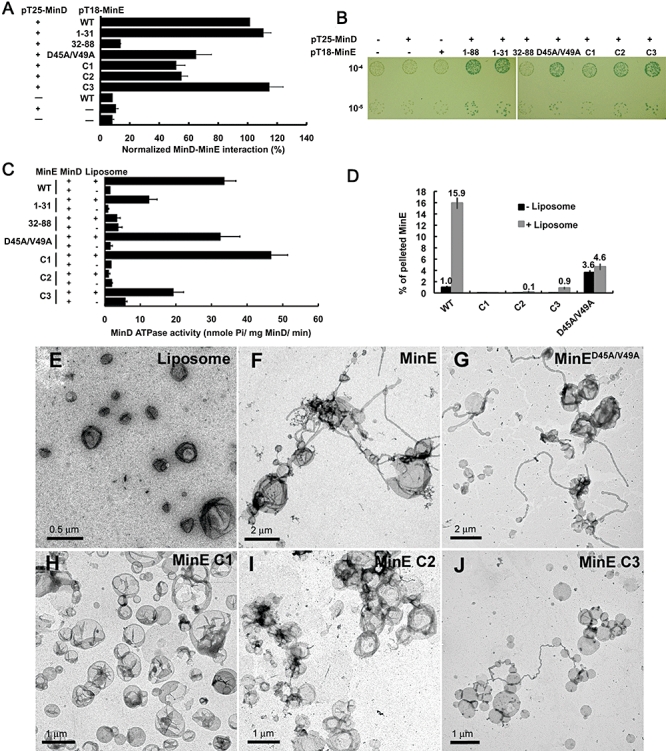
Abilities of the wild-type and mutant MinE proteins to interact with MinD, to stimulate MinD ATPase activity and to deform liposomes in vitro. A and B. The interaction between MinD and MinE was assayed using the bacterial two-hybrid system. Both liquid and plate assays are shown. Two dilutions (10−4, 10−5) of each culture were spotted on the same indicator plate (B). C. Stimulation of MinD ATPase activity by various MinE mutants in the presence or absence of liposomes. D. The full-length MinE carrying mutations in the N-terminal domain or in D45 and V49 did not co-sediment with liposomes. E–J. Degree of liposome deformation associating with the wild-type and mutant MinE proteins (C1, C2, C3 and D45A/V49A) was examined under EM.
