Abstract
Japanese morning glory (Ipomoea nil) is a representative plant lacking a yellow-flowered cultivar, although a few wild Ipomoea species contain carotenoids in their petals such as Ipomoea sp. (yellow petals) and I. obscura (pale-yellow petals). In the present study, carotenoid composition and the expression patterns of carotenogenic genes during petal development were compared among I. nil, I. obscura, and Ipomoea sp. to identify the factors regulating carotenoid accumulation in Ipomoea plant petals. In the early stage, the carotenoid composition in petals of all the Ipomoea plants tested was the same as in the leaves mainly showing lutein, violaxanthin, and β-carotene (chloroplast-type carotenoids). However, in fully opened flowers, chloroplast-type carotenoids were entirely absent in I. nil, whereas they were present in trace amounts in the free form in I. obscura. At the late stage of petal development in Ipomoea sp., the majority of carotenoids were β-cryptoxanthin, zeaxanthin, and β-carotene (chromoplast-type carotenoids). In addition, most of them were present in the esterified form. Carotenogenic gene expression was notably lower in I. nil than in Ipomoea sp. In particular, β-ring hydroxylase (CHYB) was considerably suppressed in petals of both I. nil and I. obscura. The CHYB expression was found to be significantly high in the petals of Ipomoea sp. during the synthesis of chromoplast-type carotenoids. The expression levels of carotenoid cleavage genes (CCD1 and CCD4) were not correlated with the amount of carotenoids in petals. These results suggest that both I. obscura and I. nil lack the ability to synthesize chromoplast-type carotenoids because of the transcriptional down-regulation of carotenogenic genes. CHYB, an enzyme that catalyses the addition of a hydroxyl residue required for esterification, was found to be a key enzyme for the accumulation of chromoplast-type carotenoids in petals.
Keywords: β-ring hydroxylase, carotenoid, esterification, gene expression, Ipomoea, petal colour
Introduction
Carotenoids are synthesized in chloroplasts and are essential for protecting tissues against photo-oxidative damage in the green tissues of higher plants (Britton, 1998). In flowers, carotenoids synthesized in the chromoplasts provide colour to the petals, ranging from yellow to red, in order to attract pollinators (Grotewold, 2006; Tanaka et al., 2008). The colour of a flower is an important character that determines the commercial value of ornamental plants. Although abundant flower colour of Japanese morning glory (I. nil) can be found, flowers of I. nil accumulate no carotenoids and lack a yellow-flowered cultivar. Despite a long history of attempts, crossbreeding aimed at producing yellow-flowered cultivars of I. nil has never succeeded. On the other hand, the closely related Ipomoea sp. and I. obscura have carotenoid-derived yellow and pale-yellow flowers, respectively. Hence, studying the regulatory mechanisms underlying carotenoid accumulation in Ipomoea plants at the molecular level will help in producing yellow-flowered cultivars by plant transformation.
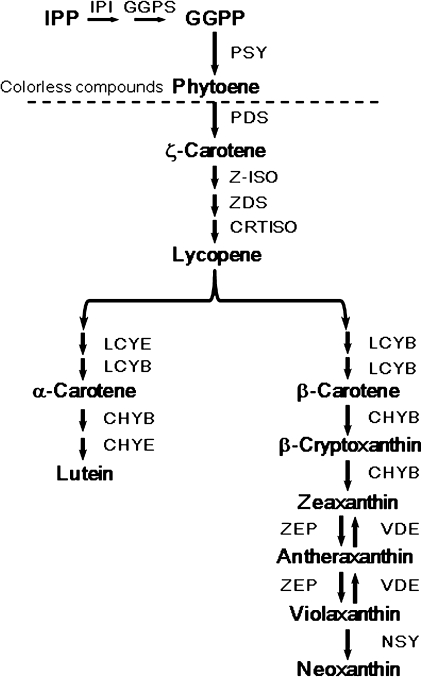
Carotenoid biosynthesis starts with one isoprene unit, C5 isopentenyl pyrophosphate (IPP; Fig. 1). Four IPPs are condensed to form C20 geranylgeranyl pyrophosphate (GGPP), and two GGPP molecules are converted to phytoene, the first C40 carotenoid, in a reaction catalysed by phytoene synthase (PSY). Phytoene is then converted via ζ-carotene to lycopene by the addition of conjugated double bonds and the conversion of cis- to trans-configurations. These reactions are catalysed by phytoene desaturase (PDS), ζ-carotene desaturase (ZDS), carotenoid isomerase (CRTISO), and 15-cis-ζ-CRTISO (Z-ISO; Li et al., 2007). The cyclization of the linear carotenoid lycopene catalysed by lycopene β-cyclase (LCYB) and/or lycopene ϵ-cyclase (LCYE) is a branch point in the pathway, leading to carotenoids with one ϵ- and one β-ring (α-carotene and its derivatives; ϵ,β-carotenoids) or two β-rings (β-carotene and its derivatives; β,β-carotenoids) (Cunningham et al., 1996; Cunningham and Gantt, 2001). Subsequently, α-carotene and β-carotene are modified by hydroxylation, epoxidation, or isomerization to express a variety of structural features. Hydroxylations of the ϵ- and β-rings of ϵ,β-carotenoids are catalysed by two haem-containing cytochrome P450 mono-oxygenases, CYP97C1 (CHYE/LUT1) and CYP97A3 (CHYB/LUT5), respectively, and α-carotene is converted to lutein (Tian et al., 2004; Kim and DellaPenna, 2006). Hydroxylation of the β-ring of β,β-carotenoids is catalysed by β-hydroxylase (CHYB; non-haem di-iron mono-oxygenase), and β-carotene is converted via β-cryptoxanthin to zeaxanthin (Britton, 1998; Cunningham and Gantt, 1998). Epoxidation of the β-ring of zeaxanthin, catalysed by zeaxanthin epoxidase (ZEP), yields violaxanthin. Violaxanthin is converted to neoxanthin by neoxanthin synthase (NSY). The oxygenated derivatives of carotene are called xanthophylls. In many cases, the majority of the carotenoids accumulated in flowers are xanthophylls. They are contained in chromoplasts in the esterified form (Hansmann and Sitte, 1982; Breithaupt and Bamedi, 2001).
Fig. 1.
Schematic of the carotenoid biosynthesis pathway in plants. IPP, isopentenyl pyrophosphate; IPI, IPP isomerase; GGPP, geranylgeranyl pyrophosphate; GGPS, GGPP synthase; PSY, phytoene synthase; PDS, phytoene desaturase; Z-ISO, 15-cis-ζ-CRTISO; ZDS, ζ-carotene desaturase; CRTISO, carotenoid isomerase; LCYE, lycopene ϵ-cyclase; LCYB, lycopene β-cyclase; CHYE, ϵ-ring hydroxylase; CHYB, β-ring hydroxylase, ZEP, zeaxanthin epoxidase; VDE, violaxanthin deepoxidase; NSY, neoxanthin synthase.
In the past decade, nearly all of the carotenogenic genes in plants have been identified (Cunningham and Gantt, 1998; Hirschberg, 2001; Howitt and Pogson, 2006). However, the mechanisms that control carotenoid biosynthesis and accumulation in plants are largely unknown (Britton et al., 2004).
Several different ways to control carotenoid accumulation in plant tissues have been reported. First, the carotenoid content depends on the plant's ability to synthesize carotenoids in the tissue. For example, white or pale-yellow cultivars or mutants of tomato (Solanum lycopersicum) fruits, canola (Brassica napus) seeds, and marigold (Tagetes erecta) petals show a lower expression of PSY than do the petals of yellow cultivars, and the transcript level is proportionate to the level of carotenoids (Fray and Grierson, 1993; Shewmaker et al., 1999; Moehs et al., 2001). The other mechanisms whereby carotenoid accumulation is regulated involve tissues that can synthesize carotenoids but contain trace amounts of carotenoids. One mechanism is focused on carotenoid degradation, and the other, on the sink capacity of carotenoids. In chrysanthemums (Chrysanthemum morifolium Ramat.), there is no significant difference between the white and yellow petals with respect to the expression levels of the carotenogenic genes (Kishimoto and Ohmiya, 2006). Synthesized carotenoids are subsequently degraded into colourless compounds by petal-specific carotenoid cleavage dioxygenase (CmCCD4a); this results in the white petal colour (Ohmiya et al., 2006). The importance of sink capacity for carotenoid accumulation was first demonstrated in the Orange (Or) mutant in cauliflower (Brassica oleracea). Transformation of the Or gene into wild-type cauliflower (or) triggers the up-regulation of the plastid fusion and/or translocation factor (Pftf) and the differentiation of proplastids and other uncoloured plastids into chromoplasts; the colour of the curd tissue changes from white to orange with an increase in the levels of β-carotene (Li et al., 2001; Paolillo et al., 2004; Lu et al., 2006).
In the present study, the patterns of carotenoid accumulation and the expression of genes related to carotenoid accumulation were compared during petal development of Ipomoea sp., I. obscura, and I. nil in order to clarify the factor that determines carotenoid accumulation in the petals of Ipomoea plants.
Materials and methods
Plant materials
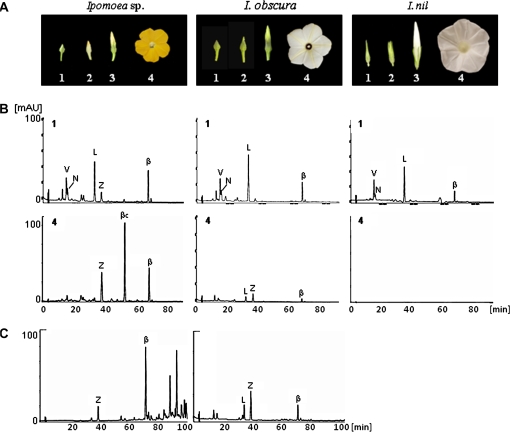
Yellow-flowered Ipomoea sp. (lineage numbers of National BioResource Project [NBRP]: Q1111), pale-yellow-flowered I. obscura (Q1114), and white-flowered cultivars of I. nil (Q0260, Q0261, Q0262, Q0263, Q0686, Q1095, Q1211) were grown under a 13/11 h light/dark photoperiod in a controlled chamber at the National Institute of Floricultural Science (Tsukuba, Ibaraki, Japan). Mature leaves were used for the analysis of carotenoid composition (Fig. 2). Petal development was divided into stages 1–4 (Fig. 3A). Petals of I. nil were almost fully open when the lights were turned on. Stages 1, 2, and 3 refer to 96, 48, and 12 h before flower opening, respectively, and stage 4 indicates fully opened flowers. Because there was a variation in the flowering time of Ipomoea sp. and I. obscura, the developmental stage was divided according to the length of the petals. The lengths of petals of Ipomoea sp. and I. obscura were c. 3–5 mm at stage 1, c. 8–10 mm at stage 2, and c. 13–15 mm at stage 3. Stage 4 indicates fully opened flowers.
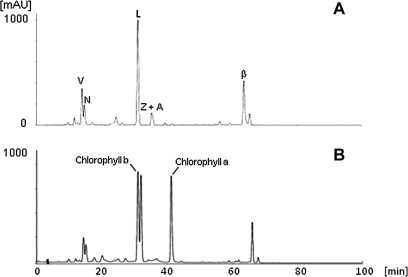
Fig. 2.
Carotenoid analysis in leaves of Ipomoea plants. Saponified (A) and non-saponified (B) carotenoids extracted from 0.1 g fresh weight (FW) of leaves of Ipomoea sp. were analysed by HPLC. V, violaxanthin; N, neoxanthin; L, lutein; Z, zeaxanthin; A, antheraxanthin; β, β-carotene.
Fig. 3.
Changes in carotenoid composition during petal development in Ipomoea plants. (A) Photographs of flowers at stages 1 and 4. HPLC elution profiles of saponified (B) and non-saponified (C) carotenoids extracted from petals of each species at various stages. V, violaxanthin; N, neoxanthin; L, lutein; Z, zeaxanthin; βc, β-cryptoxanthin; β, β-carotene. 1 and 4 indicate the sampling stages in (A).
Carotenoid extraction and HPLC analysis
Carotenoids were extracted from leaves and petals and were analysed by HPLC, according to a method previously described by Kishimoto et al. (2007). The contents were calculated according to the total peak area of HPLC chromatograms at a wavelength of 450 nm and are expressed as lutein equivalents [μg g−1 fresh weight (FW)] of the tissue.
Isolation of total RNA and synthesis of cDNA
Total RNA was isolated from petals at each stage by using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and treated with DnaseI (Invitrogen). cDNA was synthesized from total RNA (2.5 μg) by the use of the SuperScript First-Strand Synthesis System (Invitrogen) and random hexamer primers.
Cloning of cDNAs encoding carotenogenic enzymes
Degenerate primers allowing amplification were designed based on sequences corresponding to highly conserved peptide regions of isopentenyl pyrophosphate isomerase (IPI), geranylgeranyl pyrophosphate synthase (GGPS), PSY, PDS, ZDS, CRTISO, LCYB, LCYE, CHYB, and carotenoid cleavage dioxygenase 1 and 4 (CCD1 and CCD4). The cDNAs encoding these proteins were amplified by PCR using the degenerate primers. cDNAs synthesized from Ipomoea sp. and I. nil Q1211 at stage 3 were used as PCR templates. Amplified PCR products of appropriate length were cloned into the pCR2.1 vector (TA Cloning Kit, Invitrogen) and were sequenced with a Big Dye Terminator v3.1 Cycle Sequencing Kit and an ABI PRISM 3100 Genetic Analyser (Applied Biosystems, Foster City, CA, USA).
Rapid amplification of cDNA ends (RACE) was performed to obtain the 3′ and 5′ ends of the genes from petals at stage 3 of Ipomoea sp. with the SMART RACE cDNA Amplification Kit (Clontech, Palo Alto, CA, USA) according to the manufacturer's instructions. Full-length cDNA sequences encoding two isoprenoid biosynthesis enzymes (IPI, AB499048; and GGPS, AB499049) and seven carotenoid biosynthesis enzymes (PSY, AB499050; PDS, AB499051; ZDS, AB499052; CRTISO, AB499053; LCYE, AB499054; LCYB, AB499055; and CHYB, AB499056), and partial-length cDNA sequences encoding carotenoid cleavage enzymes (CCD1, AB499060 and CCD4, AB499059) are available in the GenBank nucleotide database (see Supplementary Table S1 at JXB online).
Quantitative real-time PCR analysis
The transcript levels of IPI, GGPS, PSY, PDS, ZDS, CRTISO, LCYE, LCYB, CHYB, CCD1, and CCD4 were analysed using quantitative real-time PCR (RT-qPCR) with the SYBR Premix Ex Taq II polymerase (TaKaRa, Ohtsu, Japan) and LightCycler System (Roche Diagnostics, Basel, Switzerland), according to the manufacturers’ instructions. Each reaction (final volume, 20 μl) consisted of 10 μl 2× SYBR Premix Ex Taq II (TaKaRa), 0.5 μM each of the forward and reverse primers, and 2 μl of the cDNA template (corresponding to 50 ng of total RNA). The reaction mixtures were heated to 95 °C for 20 s, followed by 50 cycles at 95 °C for 5 s and 60 °C for 20 s. A melting curve was generated for each sample at the end of each run to ensure the purity of the amplified products. The gene-specific primers for PCR were designed using the conserved sequences among Ipomoea plants used in the experiments (Table 1). The actin primers were designed using the cDNA sequences of I. nil actin 4 (AB054978; Yamada et al., 2007). Relative standard curves describing the PCR efficiencies for each primer pair were calculated by the following equation (PCR efficiency=10–1/slope–1), as described by Bustin et al. (2009). Each assay using the gene-specific primers amplified a single product of correct size with high PCR efficiency (>90%). The expression levels of actin and elongation factor 1-α were used to normalize the transcript levels of each sample. The expression patterns after normalization using actin or elongation factor 1-α as the reference gene were similar (data not shown); however, only data normalized with actin have been included in this paper. The plasmid solution containing each gene was serially diluted 10-fold (from 108 molecules μl−1 to 103 molecules μl−1) and used for a standard curve assay. The transcript levels are given as the copy number per 50 ng of total RNA. The linear dynamic ranges cover at least four orders of magnitude and the level of transcripts in each reaction mixture was within the range (data not shown).
Table 1.
Primer pairs used for real-time PCR
| Gene | Direction | Sequence (5′→3′) |
| IPI | Forward | TCATTGTGCGGGATGTCAGC |
| Reverse | GCGGCTTCCTTGAGAGTCCC | |
| GGPS | Forward | GGCGATTCTCACCAAGGAGC |
| Reverse | CTTCCAGCGCCTTGTTCACC | |
| PSY | Forward | GTGCAGAGTATGCAAAGACG |
| Reverse | GCCTAGCCTCCCATCTATCC | |
| PDS | Forward | CCGCCCTTTGAAGGTAGTTT |
| Reverse | GTGGCCAGCATCTGCTAAAT | |
| ZDS | Forward | TTCCTATTGGAGCACCCTTG |
| Reverse | ACGAATGTCCCTCATTGCTC | |
| CRTISO | Forward | ACCTTGCTCGTGACAGTGG |
| Reverse | CAGCAACACGATGAGCACAC | |
| LCYE | Forward | ATGGTGTGGAGGTTGAGGTG |
| Reverse | ACCAAACAAGTTTCCTCAAA | |
| LCYB | Forward | ATAGAGAGGAGGCGGCAAAG |
| Reverse | GAAACAGCCGGGATGATAGA | |
| CHYB | Forward | CCTATCGCCGACGTACCTTA |
| Reverse | TCGTTTAGCCCACCAACTTC | |
| CCD4 | Forward | CGTGGGCCTTACCATCTTTT |
| Reverse | AAACGTTGGGGATAACAGGAG | |
| CCD1 | Forward | GGCTCGCTTTGGAGTCCTTC |
| Reverse | TCATCTCCCTCCTCCCATGC |
Statistically significant differences with respect to each developmental stage for values were determined by Tukey–Kramer test at the 5% level.
Results
Changes in carotenoid composition during petal development
HPLC chromatograms of the carotenoid extracts obtained from the leaves of Ipomoea sp., I. obscura, and I. nil were similar. A representative chromatogram of Ipomoea sp. is shown in Fig. 2A. The majority of the carotenoids in leaves were lutein, violaxanthin, and β-carotene, which are essential for photosynthesis. Carotenoids in the non-saponified extract from leaves exhibited an HPLC chromatogram similar to those in the saponified leaf extract, except that chlorophyll a and chlorophyll b were detected in the non-saponified extract (Fig. 2B). The carotenoid composition in leaves was designated ‘chloroplast-type carotenoid’. The total carotenoid content in the leaves of all tested cultivars was around 300 μg g−1 FW.
Changes in the HPLC chromatograms of carotenoid extracts during petal development in Ipomoea plants are shown in Fig. 3B and C and Table 2. At stage 1, all petals tested were pale green and showed the same chromatograms as chloroplast-type carotenoids, mainly showing lutein, violaxanthin, and β-carotene, albeit at lower levels than in leaves (<10 μg g−1 FW). In petals of I. nil at stage 4, the carotenoid content decreased below the detection limit, whereas small amounts of chloroplast-type carotenoids remained in I. obscura, and the carotenoids existed in the free form just as in leaves. In petals of Ipomoea sp. at stage 4, the carotenoid composition (designated ‘chromoplast-type carotenoid’) was completely different from the chloroplast-type carotenoids: lutein and violaxanthin levels were drastically reduced, and approximately 85% of the total carotenoids were made up of β-cryptoxanthin, zeaxanthin, and β-carotene. In addition, xanthophylls such as β-cryptoxanthin and zeaxanthin existed in the esterified form (Fig. 3C).
Table 2.
Concentrations of carotenoid compounds in Ipomoea leaves and petals
| Organ | Violaxanthin | Neoxanthin | Lutein | Zeaxanthin | β-cryptoxanthin | β-carotene | |
| Ipomoea sp. | |||||||
| Leaf | 39.52±8.87 | 26.17±4.15 | 148.92±12.33 | 28.80±3.57 | ND | 89.45±10.72 | |
| Flower | Stage 1 | 1.96±0.48 | 1.14±0.30 | 3.73±0.66 | 0.98±0.54 | ND | 2.50±0.30 |
| Stage 2 | 5.93±1.19 | 10.56±1.35 | 11.27±0.91 | 36.65±7.58 | 17.98±7.64 | 15.71±5.44 | |
| Stage 3 | 1.78±0.33 | 4.04±3.07 | 3.57±1.75 | 41.40±4.99 | 32.94±3.66 | 11.57±4.61 | |
| Stage 4 | 2.84±1.59 | 5.19±2.27 | 3.61±2.26 | 34.14±7.16 | 43.25±4.60 | 17.71±5.10 | |
| I. obscura | |||||||
| Leaf | 50.73±4.28 | 40.63±10.96 | 164.81±14.40 | 37.16±8.33 | ND | 62.97±10.74 | |
| Flower | Stage 1 | 1.28±0.27 | 1.08±0.18 | 3.73±1.15 | 0.72±0.63 | ND | 1.43±.0.56 |
| Stage 2 | 2.66±0.45 | 1.23±0.49 | 4.41±0.63 | 1.81±0.53 | ND | 3.15±0.56 | |
| Stage 3 | 1.70±0.35 | 1.12±0.42 | 2.56±1.07 | 1.61±1.03 | ND | 2.05±1.05 | |
| Stage 4 | 0.52±0.34 | 0.46±0.26 | 1.00±0.13 | 1.11±0.13 | ND | 0.22±0.03 | |
| I. nil | |||||||
| Leaf | 34.92±9.55 | 19.52±1.52 | 129.03±19.50 | 19.94±10.68 | ND | 61.22±11.13 | |
| Flower | Stage 1 | 3.10±0.82 | 0.71±0.10 | 3.65±1.10 | 0.64±0.18 | ND | 1.45±0.69 |
| Stage 2 | 2.83±0.63 | 1.20±0.65 | 4.97±0.66 | 0.98±0.44 | ND | 1.03±0.06 | |
| Stage 3 | 0.81±0.12 | 0.17±.03 | 0.79±0.08 | 0.24±0.09 | ND | 0.22±0.03 | |
| Stage 4 | ND | ND | ND | ND | ND | ND |
Values are expressed as μg g−1 FW; ND, not detectable; FW, fresh weight. Each value represents the mean result from triplicate ±SD
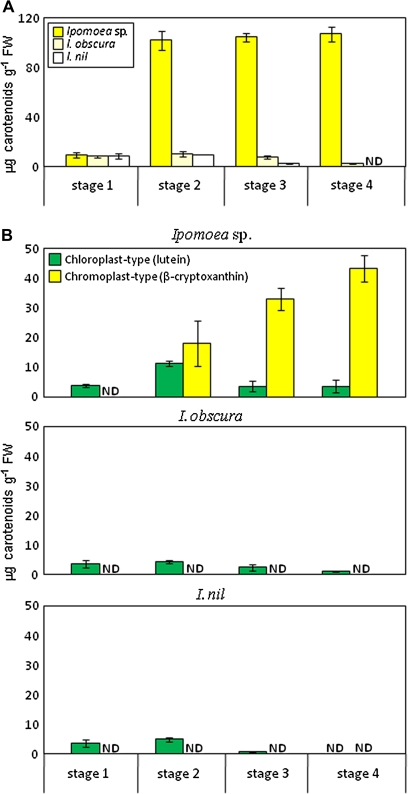
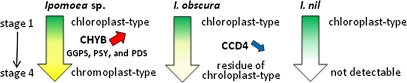
Figure 4 shows changes in the amounts of total carotenoids and chloroplast- and chromoplast-type carotenoids during petal development in Ipomoea plants. Chloroplast-type carotenoids are represented by lutein because lutein is the most abundant carotenoid in the leaves and is contained in only a trace amount in petals (Table 2). Similarly, chromoplast-type carotenoids are represented by β-cryptoxanthin. From stage 2 to stage 4, petals of Ipomoea sp. showed dramatic component changes in the carotenoids: chloroplast-type carotenoids were replaced by chromoplast-type carotenoids. On the other hand, petals of I. obscura and I. nil at stage 2 retained chloroplast-type carotenoids. In petals of I. obscura at stage 4, small amount of chloroplast-type carotenoids remained because of the slow decrease in the carotenoid level, but chromoplast-type carotenoids were not detected. In I. nil, the carotenoid content drastically decreased from stage 3 onwards and was entirely absent at stage 4. These results indicate that the yellow petal colour of Ipomoea sp. is due to newly synthesized chromoplast-type carotenoids, whereas the pale-yellow petal colour of I. obscura is due to the remaining chloroplast-type carotenoids synthesized at the early stage of petal development.
Fig. 4.
Changes in carotenoid composition during petal development. Total carotenoid concentration (A) and chloroplast- and chromoplast-type carotenoid content (B) in petals of Ipomoea sp., I. obscura, and I. nil at stages 1 to 4. Chloroplast- and chromoplast-type carotenoids are represented by lutein and β-cryptoxanthin, respectively. Each bar represents the mean result from triplicate ±SD. ND, not detectable.
Analysis of carotenogenic gene expression during petal development
Genes encoding enzymes involved in isoprenoid and carotenoid biosyntheses were isolated and sequenced (see Supplementary Table S1 at JXB online). On the basis of the gene sequences, gene-specific primers were designed (Table 1), and the carotenogenic gene expressions were quantified by RT-qPCR using the primers. The differences in the amounts of total RNA were normalized with respect to the levels of actin mRNA in each sample (Figs 5, 6).
Fig. 5.
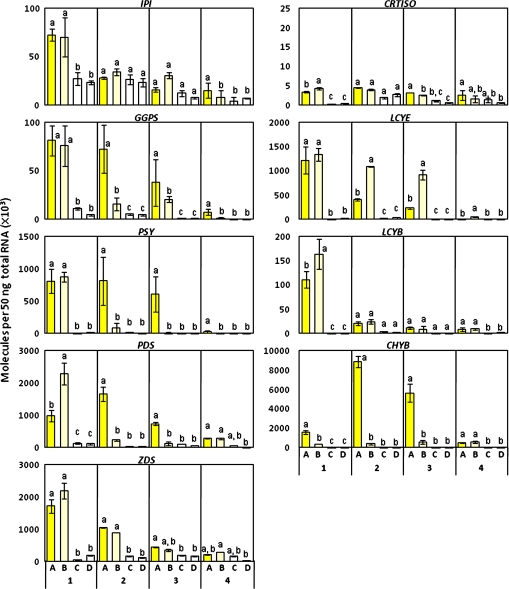
Changes in carotenogenic gene expression during petal development. RT-qPCR analyses were performed in triplicate by using gene-specific primers, and the expression levels were normalized against mRNA levels of actin; mean values (SE) are shown. 1, 2, 3, and 4 indicate sampling stages. (A) Ipomoea sp. (Q1111); (B) I. obscura (Q1114); (C) I. nil (Q0686); (D) I. nil (Q1211). IPI, IPP isomerase; CRTISO, carotenoid isomerase; GGPS, geranylgeranyl pyrophosphate synthase; LCYE, lycopene ϵ-cyclase; PSY, phytoene synthase; LCYB, lycopene β-cyclase; PDS, phytoene desaturase; CHYB, β-ring hydroxylase; ZDS, ζ-carotene desaturase. Different letters within each stage indicate significant differences by Tukey-Kramer test (P <0.05).
Fig. 6.
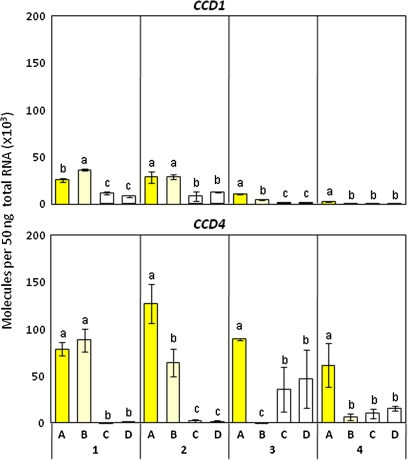
Changes in the expression pattern of CCD1 and CCD4 during petal development. RT-qPCR analyses were performed in triplicate by using gene-specific primers, and the expression levels were normalized against mRNA levels of actin; mean values (SE) are shown. Sampling stages and plant materials are the same as described in Fig. 5. Different letters within each stage indicate significant differences by Tukey–Kramer test (P <0.05).
The transcript levels of most genes in Ipomoea sp. and I. obscura were similar at stage 1 (Fig. 5). At stages 2 and 3, the transcript levels of geranylgeranyl pyrophosphate synthase (GGPS), PSY, and PDS drastically declined in I. obscura but remained high in Ipomoea sp. Among the carotenogenic genes tested, only CHYB showed a significant increase in the petals of Ipomoea sp. as the carotenoid composition changed from the chloroplast-type to the chromoplast-type. By contrast, the expression of CHYB was very low throughout petal development in I. obscura. In both Ipomoea sp. and I. obscura, the expression levels of all genes were lower at stage 4 than those during the early stage of petal development.
All seven white-flowered cultivars tested of I. nil showed similar expression patterns. The results of two representative cultivars (Q0686, and Q1211) are shown in Fig. 5. All carotenogenic genes, except CHYB, were expressed at stages 1 and 2 in petals of I. nil, albeit at low levels. At stages 3 and 4, the expressions of most genes were down-regulated. In particular, the CHYB transcript level was extremely low in I. nil at all stages.
Analysis of the expression levels of genes involved in caroteniod cleavage
To examine whether carotenoid degradation is involved in the formation of the white colour in Ipomoea petals, the transcript level of genes encoding carotenoid cleavage dioxygenases (CCDs) were analysed by RT-qPCR (Fig. 6). Among the four types of CCD homologues, CCD1, CCD4, CCD7, and CCD8, reported in Arabidopsis (Tan et al., 2003), it was possible to obtain cDNAs encoding CCD1 and CCD4 homologues from Ipomoea plants.
All the Ipomoea plants tested showed a low level of CCD1 expression during the course of petal development. The expression pattern of CCD4 during petal development differed among the Ipomoea plants. CCD4 was expressed at a relatively high level in petals of Ipomoea sp. throughout development. In I. obscura, wherein petals at stage 4 contained chloroplast-type carotenoids, CCD4 expression declined remarkably during petal development. By contrast, CCD4 expression remained high at stages 3 and 4 in I. nil. These results suggest that the pale-yellow petal colour of I. obscura derived from chloroplast-type carotenoids is caused by a reduced level of CCD4 expression.
Discussion
Changes in carotenoid composition during petal development
The leaves of most plants show similar carotenoid profiles, containing both ϵ,β-carotenoids and β,β-carotenoids (Goodwin and Britton, 1988). Carotenoids essential for photosynthesis, such as lutein, violaxanthin, and β-carotene, are generally found in leaves. Because they are contained in chloroplasts, these were designated as ‘chloroplast-type carotenoids.’ By contrast, the carotenoid composition in flowers varies depending on plant species, and carotenoids accumulating in chromoplasts are in a more oxidized form than chloroplast-type carotenoids (Deli et al., 1988; Goodwin and Britton, 1988; Kull and Pfander, 1997; Maoka et al., 2000; Tai and Chen, 2000; Kishimoto et al., 2005). The present results show that in all plants tested, petals contained chloroplast-type carotenoids at the early developmental stage (Fig. 3). At the late stage of petal development, the levels of chloroplast-type carotenoids decreased, and high levels of carotenoids, of which the composition differed from that of chloroplast-type carotenoids, accumulated only in Ipomoea sp.: the accumulated carotenoids included β,β-carotenoids such as β-cryptoxanthin, zeaxanthin, and β-carotene (designated as ‘chromoplast-type carotenoids’). In addition, the chromoplast-type carotenoids were mostly present in the esterified form, while the chloroplast-type carotenoids existed in the free form. The importance of esterification for carotenoid sequestration into chromoplasts has been well documented in pepper fruits (Minguez-Mosquera and Hornero-Mendez, 1994a, b; Hornero-Mendez and Minguez-Mosquera, 2000). Xanthophyll esterification was accompanied by the transition of chloroplasts (green fruits) into chromoplasts (red fruits). Previous studies have shown that, in flowers of marigold and Eustoma, a significant proportion of carotenoids are esterified during petal development (Moehs et al., 2001; Nakayama et al., 2006). Hence, it is assumed that esterification is an important process for the sequestration of carotenoids into chromoplasts in Ipomoea sp.
Differences in carotenogenic gene expression during petal development
When the carotenogenic gene expression in petals of Ipomoea plants was analysed in relation to compositional changes in carotenoids occurring during petal development, the most prominent feature was the difference in CHYB expression among Ipomoea sp., I. obscura, and I. nil (Fig. 5). CHYB expression drastically increased at stages 2 and 3 in petals of Ipomoea sp., whereas it remained extremely low throughout the course of petal development both in I. obscura and I. nil. There is increasing evidence that CHYB plays an important role in carotenoid accumulation in the chromoplast of various plants. The wf mutant in tomato, which produces the white flower phenotype is caused by a mutation in CrtR-b2, a CHYB homologue that is specifically expressed in petals (Galpaz et al., 2006). The wf mutant shows a significant decrease in the levels of β-carotene derivatives in petals, whereas the transcript levels of genes encoding the rate-limiting enzymes involved in isoprenoid and carotenoid biosyntheses, such as 1-deoxy-D-xylulose-5-phosphate synthase (DXS), PSY, GGPS, PDS, and ZDS show the same transcript levels as in the petals of wild-type plants. In tepals of Asiatic hybrid lily (Lilium spp.), the expression level of the CHYB homologue in various cultivars correlated with the carotenoid content and more closely than any other carotenogenic genes (Yamagishi et al., 2009). In Arabidopsis, the homologue of CHYB was not expressed in the floral organ (Kim et al., 2009). Hydroxylation is an important process for the esterification of carotenoids, which in turn is necessary for carotenoid sequestration and stabilization in the chromoplast. The up-regulation of CHYB, which encodes for an enzyme that catalyses the addition of hydroxyl residues required for esterification, may be a key event in carotenoid accumulation in the chromoplast.
Generally, it is thought that the apportionment of substrates into the pathways leading to the synthesis of α- and β-carotenoids is determined simply by the relative amounts or activities of LCYB and LCYE. For example, in chrysanthemums, the difference in carotenoid components between petals and leaves is caused by the different expression levels of LCYB and LCYE (Kishimoto and Ohmiya, 2006). In addition, the repression of LCYE expression increases the β,β-carotenoid content in potato tubers and canola seeds (Diretto et al., 2006; Yu et al., 2008). In Ipomoea sp., the decrease in the levels of chloroplast-type carotenoids is accompanied by a decrease in the level of LCYE transcripts. The level of LCYB transcripts, however, showed no correlation with the accumulation of chromoplast-type carotenoids (mainly β,β-carotenoids) and did not considerably differ from the LCYB transcript level in I. obscura. These results suggest that CHYB and not LCYB functions as a key enzyme in the synthesis of chromoplast-type carotenoids in the petals of Ipomoea sp.
Expression patterns of genes involved in carotenoid degradation during petal development
In chrysanthemums, CmCCD4a contributes to the white colour of petals by cleaving carotenoids into colourless compounds (Ohmiya et al., 2006). However, little data on whether the same mechanism is applicable to other plant species are available. In petals of Ipomoea sp., the CCD4 expression was relatively high at stages 2 and 3 when the level of chromoplast-type carotenoids was increased. In addition, both Ipomoea sp. and I. nil showed high levels of expression of CCD4 during the degradation of chloroplast-type carotenoids. In petals of I. obscura, which accumulate chloroplast-type carotenoids even at stage 4, CCD4 drastically decreased as the petals matured. These results suggest that in Ipomoea plants, CCD4 is not involved in the degradation of chromoplast-type carotenoids but is involved in the degradation of chloroplast-type carotenoids.
To examine whether the carotenoid degradation mechanism is responsible for white colour formation in petals of Ipomoea plants, Ipomoea sp. and I. obscura were crossed (see Supplementary Fig. S1 at JXB online). It is thought that the white petal colour would be dominant over the yellow colour if carotenoid degradation is responsible for the white colour formation of petals in Ipomoea. However, the petals of all progenies obtained by the crossing were yellow and accumulated chromoplast-type carotenoids, suggesting that carotenoid cleavage is not responsible for the lack of chromoplast-type carotenoids in I. obscura.
The sink capacity of carotenoids is another factor to be considered. The importance of sink capacity for carotenoid accumulation was first demonstrated in cauliflower Or mutants (Lu et al., 2006). Transformation of the Or gene into wild-type cauliflower (or) exhibit increased expression of Pftf, but not of carotenoid biosynthesis genes (Li et al., 2001; Lu et al., 2006). The colour of the curd tissue of transformants changes from white to orange with an increase in the levels of β-carotene. In Ipomoea plants, however, there was no significant correlation between the level of Pftf transcripts and the carotenoid content in petals (data not shown).
These results suggest that the carotenoid content in petals of Ipomoea plants is related neither to carotenoid degradation activity nor to the sink capacity of carotenoids, but instead to the transcriptional down-regulation of carotenogenic genes, especially CHYB.
Putative mechanism for the regulation of carotenogenic gene expression in Ipomoea petals
In the present study, it is shown that petals of I. nil and I. obscura accumulated little, if any, chromoplast-type carotenoids because of the transcriptional down-regulation of carotenogenic genes, in particular, the transcriptional down-regulation of CHYB. Two hypotheses were postulated to explain such down-regulation. (i) A chromoplast-specific carotenoid biosynthesis pathway exists in Ipomoea sp. but not in I. nil or I. obscura. (ii) Petal-specific down-regulation of the carotenoid biosynthesis pathway in I. nil and I. obscura causes low carotenoid production.
Reports of genes encoding chromoplast-specific carotenoid biosynthesis enzymes suggest a chromoplast-specific carotenoid biosynthesis pathway (Bartley et al., 1992; Fray and Grierson, 1993; Pecker et al., 1996; Ronen et al., 2000). Two types of CtrR-b genes, which encode non-haem β-carotene hydroxylase, exist in tomato and pepper, one of which is specifically expressed in the fruit (Bouvier et al., 1998; Galpaz et al., 2006). However, in this study, only one type of CHYB gene was isolated from Ipomoea sp. The predicted polypeptide sequence of CHYB in Ipomoea sp. shares 87% similarity with both tomato CRTR-B1 (chloroplast-specific CHYB) and CRTR-B2 (chromoplast-specific CHYB) (see Supplementary Fig. S2 at JXB online). Because the chloroplast transit peptide (cTP) sequences of CRTR-B1 and CRTR-B2 shared only 56% similarity, the cTP sequence of CHYB in Ipomoea sp. was compared with that of tomato CRTR-B1 and CRTR-B2. The cTP of Ipomoea CHYB shared 56% and 59% identity with the cTP sequences of tomato CRTR-B1 and CRTR-B2, respectively (see Supplementary Fig. S2 at JXB online). CHYB cannot be categorized as a CRTR-B1 type or a CRTR-B2 type on the basis of its sequence; hence, it is assumed that CHYB has a redundant function in Ipomoea plants. The existence of chromoplast-specific PSY and LCYB has also been reported previously (Fray and Grierson, 1993; Pecker et al., 1996). However, only one type each of PSY and LCYB was isolated from Ipomoea sp. Moreover, the EST database of I. nil holds only one contig for CHYB, PSY, and LCYB (http://ipomoeanil.nibb.ac.jp/). Although the possibility cannot be excluded that genes encoding chromoplast-specific enzymes exist in Ipomoea plants, the formation of white petal colour in Ipomoea plants may be controlled by a mechanism different from that in the tomato wf mutant.
It is well known that the carotenoid content in plant tissues, especially flowers and fruits, varies widely within the same species. In marigold, differences in petal colour, from pale-yellow to dark orange, are caused by different levels of lutein accumulation. In a previous study, it was found that the lutein content correlates well with the level of PSY and DXS transcripts (Moehs et al., 2001). It has also been demonstrated that PSY in tomato fruits (Pecker et al., 1992; Giuliano et al., 1993; Fraser et al., 1994) and GGPS, PSY, and PDS in pepper (Hugueney et al., 1996) are rate-limiting enzymes in carotenoid biosynthesis. The CHYB transcript level in petals of Ipomoea sp. drastically increased with an increase in chromoplast-type carotenoid accumulation at stages 2 and 3, but that of I. obscura and I. nil was significantly low throughout petal development. Moreover, at stages 2 and 3, the transcript levels of GGPS, PSY, and PDS remarkably decreased in petals of I. obscura and I. nil but remained high in those of Ipomoea sp. (Fig. 5). These results suggest that CHYB is mainly responsible for the regulation of chromoplast-specific carotenoid accumulation in petals of Ipomoea plants, and GGPS, PSY, and PDS are also involved the regulation process (Fig. 7).
Fig. 7.
Schematic representation of the mode of carotenoid accumulation in petals of Ipomoea plants. Differences in carotenoid accumulation patterns and related enzymes during petal development among Ipomoea plants are shown. CHYB, β-ring hydroxylase; GGPS, geranylgeranyl pyrophosphate synthase; PSY, phytoene synthase; PDS, phytoene desaturase; CCD, carotenoid cleavage dioxygenase.
Recent studies have shown that the transcriptional regulation of carotenoid biosynthesis enzymes is the major factor controlling carotenogenesis in plant tissues (Fraser and Bramley, 2004; Sandmann et al., 2006). One possible mechanism for the repression of multiple carotenogenic genes in petals of I. nil is feedback inhibition at the transcriptional level caused by repression of CHYB expression. In the wf mutant of tomato, the expression level of genes upstream of the carotenoid biosynthesis pathway, such as of DXS, GGPS, PSY, and PDS, is not affected by CHYB repression (Galpaz et al., 2006). Little data are available regarding feedback inhibition of the carotenoid pathway by an enzyme downstream of the pathway. Transgenic plants overexpressing CHYB will provide an answer to whether CHYB influences the expression level of upstream genes.
Another possible mechanism is down-regulation of multiple carotenogenic genes caused by a common transcription factor. In Arabidopsis, only one PSY gene exists. AtRAP2.2 was isolated as a transcription factor that binds to the cis-acting element ATCTA in the promoter of PSY. The ATCTA element is also present in the promoter of PDS, which suggests that a common regulatory mechanism exists in both genes (Welsch et al., 2007). Co-repression of multiple carotenogenic genes during petal development in I. obscura and I. nil suggests the existence of a transcription factor common to multiple carotenogenic genes; however, there are no reports on the mechanism regulating the entire carotenoid biosynthesis pathway. Understanding the mechanism underlying the regulation of carotenoid biosynthesis in plant tissues will accelerate the process of engineering this pathway, which has only partly been achieved thus far.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. Genes encoding isoprenoid and carotenoid biosynthesis enzymes isolated from Ipomoea sp.
Supplementary Fig. S1. Crossing between Ipomoea sp. and I. obscura.
Supplementary Fig. S2. Multiple sequence comparison of CHYB among various plant species.
Supplementary Material
Acknowledgments
We thank Dr Eiji Nitasaka of Kyushu University (NBRP; National BioResource Project) for providing all the Ipomoea seeds used in our experiments and for useful discussions. This work was supported in part by a Grant-in-Aid from the National Agriculture and Food Research Organization (NARO), Japan.
Glossary
Abbreviations
- CCD
carotenoid cleavage dioxygenase
- CHYB
β-ring hydroxylase
- CRTISO
carotenoid isomerase
- DXS
1-deoxy-D-xylulose-5-phosphate synthase
- GGPP
geranylgeranyl pyrophosphate
- GGPS
GGPP synthase
- IPP
isopentenyl pyrophosphate
- IPI
IPP isomerase
- LCYB
lycopene β-cyclase
- LCYE
lycopene ϵ-cyclase
- NCED
9-cis-epoxycarotenoid dioxygenase
- NSY
neoxanthin synthase
- PDS
phytoene desaturase
- Pftf
plastid fusion and/or translocation factor
- PSY
phytoene synthase
- ZDS
ζ-carotene desaturase
- ZEP
zeaxanthin epoxidase
References
- Bartley GE, Viitanen PV, Bacot KO, Scolnik PA. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. Journal of Biological Chemistry. 1992;267:5036–5039. [PubMed] [Google Scholar]
- Bouvier F, Keller Y, d'Harlingue A, Camara B. Xanthophyll biosynthesis: molecular and functional characterization of carotenoid hydroxylases from pepper fruits (Capsicum annuum L.) Biochimica et Biophysica Acta. 1998;1391:320–328. doi: 10.1016/s0005-2760(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Breithaupt DE, Bamedi A. Carotenoid esters in vegetables and fruits: a screening with emphasis on beta-cryptoxanthin esters. Journal of Agricultural and Food Chemistry. 2001;49:2064–2070. doi: 10.1021/jf001276t. [DOI] [PubMed] [Google Scholar]
- Britton G. Overview of carotenoid biosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Vol. 3. Basel: Birkhäuser; 1998. pp. 13–147. [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H. Carotenoids handbook. 2004. Basel: Birkhäuser. [Google Scholar]
- Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Jr, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Jr, Gantt E. One ring or two? Determination of ring number in carotenoids by lycopene ϵ-cyclases. Proceedings of the National Academy of Sciences, USA. 2001;98:2905–2910. doi: 10.1073/pnas.051618398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FX, Jr, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E. Functional analysis of the β and ϵ lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant Cell. 1996;8:1613–1626. doi: 10.1105/tpc.8.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli J, Molnár P, Tóth G, Szabolcs J, Radics L. Determination of the geometrical configuration of naturally occurring mono- cis-lutein epoxides. Phytochemistry. 1988;27:547–549. [Google Scholar]
- Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biology. 2006;6:13. doi: 10.1186/1471-2229-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Truesdale MR, Bird CR, Schuch W, Bramley PM. Carotenoid biosynthesis during tomato fruit development. Plant Physiology. 1994;105:405–413. doi: 10.1104/pp.105.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Molecular Biology. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- Galpaz N, Ronen G, Khalfa Z, Zamir D, Hirschberg J. A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. The Plant Cell. 2006;18:1947–1960. doi: 10.1105/tpc.105.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G, Bartley GE, Scolnik PA. Regulation of carotenoid biosynthesis during tomato development. The Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin TW, Britton G. Distribution and analysis of carotenoids. In: Goodwin TW, editor. Plant pigments. London: Academic Press; 1988. pp. 62–132. [Google Scholar]
- Grotewold E. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- Hansmann P, Sitte P. Composition and molecular structure of chromoplast globules of. Viola tricolor. Plant Cell Reports. 1982;1:111–114. doi: 10.1007/BF00272366. [DOI] [PubMed] [Google Scholar]
- Hirschberg J. Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology. 2001;4:210–218. doi: 10.1016/s1369-5266(00)00163-1. [DOI] [PubMed] [Google Scholar]
- Hornero-Méndez D, Mínguez-Mosquera MI. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplasts of Capsicum annuum ripening fruits is a constitutive process and useful for ripeness index. Journal of Biological Chemistry. 2000;48:1617–1622. doi: 10.1021/jf9912046. [DOI] [PubMed] [Google Scholar]
- Howitt CA, Pogson BJ. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell and Environment. 2006;29:435–445. doi: 10.1111/j.1365-3040.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Quennemet J, d'Harlingue A, Camara B. Developmental and stress regulation of gene expression for plastid and cytosolic isoprenoid pathways in pepper fruits. Plant Physiology. 1996;111:619–626. doi: 10.1104/pp.111.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dellapenna D. Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid β-ring hydroxylase CYP97A3. Proceedings of the National Academy of Sciences, USA. 2005;103:3474–3479. doi: 10.1073/pnas.0511207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith JJ, Tian L, Dellapenna D. The evolution and function of carotenoid hydroxylases in. Arabidopsis. Plant and Cell Physiology. 2009;50:463–479. doi: 10.1093/pcp/pcp005. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Maoka T, Sumitomo K, Ohmiya A. Analysis of carotenoid composition in petals of calendula (Calendula officinalis L.) Bioscience, Biotechnology, and Biochemistry. 2005;69:2122–2128. doi: 10.1271/bbb.69.2122. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Ohmiya A. Regulation of carotenoid biosynthesis in petals and leaves of chrysanthemum (Chrysanthemum morifolium Ramat.) Physiologia Plantarum. 2006;128:437–447. [Google Scholar]
- Kishimoto S, Sumitomo K, Yagi M, Nakayama M, Ohmiya A. Three routes to orange petal colour via carotenoid component in 9 Compositae species. Journal of the Japanese Society for Horticultural Science. 2007;76:250–257. [Google Scholar]
- Kull D, Pfander H. Isolation and structure elucidation of two (Z)-isomers of lutein from the petals of rape (Brassica napus) Journal of Agricultural and Food Chemistry. 1997;45:4201–4203. [Google Scholar]
- Li F, Murillo C, Wurtzel ET. Maize Y9 encodes a product essential for 15-cis-ζ-carotene isomerization. Plant Physiology. 2007;144:1181–1189. doi: 10.1104/pp.107.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Paolillo DJ, Parthasarathy MV, DiMuzio EM, Garvin DF. A novel gene mutation that confers abnormal patterns of β-carotene accumulation in cauliflower (Brassica oleracea var. botrytis) The Plant Journal. 2001;26:59–67. doi: 10.1046/j.1365-313x.2001.01008.x. [DOI] [PubMed] [Google Scholar]
- Lu S, Van Eck J, Zhou X, et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. The Plant Cell. 2006;18:3594–3605. doi: 10.1105/tpc.106.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoka T, Fujiwara Y, Hashimoto K, Takeda S, Takaragaki S, Ida K. A new retro-carotenoid from the petals of the Californian yellow poppy Eschscholzia californica. Journal of Natural Products. 2000;63:1288–1289. doi: 10.1021/np0000670. [DOI] [PubMed] [Google Scholar]
- Mínguez-Mosquera MI, Hornero-Méndez D. Formation and transformation of pigments during the fruit ripening of Capsicum annuum cv Bola and Agridulce. Journal of Agricultural and Food Chemistry. 1994a;42:38–44. [Google Scholar]
- Mínguez-Mosquera MI, Hornero-Méndez D. Changes in carotenoid esterification during the fruit ripening of Capsicum annuum cv. Bola. Journal of Agricultural and Food Chemistry. 1994b;42:640–644. [Google Scholar]
- Moehs CP, Tian L, Osteryoung KW, DellaPenna D. Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Molecular Biology. 2001;45:281–293. doi: 10.1023/a:1006417009203. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Miyasaka M, Maoka T, Yagi M, Fukuta N. A carotenoid-derived yellow Eustoma screened under blue and ultraviolet lights. Journal of the Japanese Society for Horticultural Science. 2006;75:161–165. [Google Scholar]
- Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white colour formation in chrysanthemum petals. Plant Physiology. 2006;142:1193–1201. doi: 10.1104/pp.106.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo DJ, Jr, Garvin DF, Parthasarathy MV. The chromoplasts of Or mutants of cauliflower (Brassica oleracea L. var. botrytis) Protoplasma. 2004;224:245–253. doi: 10.1007/s00709-004-0059-1. [DOI] [PubMed] [Google Scholar]
- Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J. A single polypeptide catalysing the conversion of phytoene to ζ-carotene is transcriptionally regulated during tomato fruit ripening. Proceedings of the National Academy of Sciences, USA. 1992;89:4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecker I, Gabbay R, Cunningham Jr, FX, Hirschberg J. Cloning and characterization of the cDNA for lycopene β-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Molecular Biology. 1996;30:807–819. doi: 10.1007/BF00019013. [DOI] [PubMed] [Google Scholar]
- Ronen G, Carmel-Goren L, Zamir D, Hirschberg J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold colour mutations in tomato. Proceedings of the National Academy of Sciences, USA. 2000;97:11102–11107. doi: 10.1073/pnas.190177497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G, Romer S, Fraser PD. Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metabolic Engineering. 2006;8:291–302. doi: 10.1016/j.ymben.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed specific overexpression of phytoene synthase increase in carotenoids and other metabolic effects. The Plant Journal. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Tai C-Y, Chen BH. Analysis and stability of carotenoids in the flowers of daylily (Hemerocallis disticha) as affected by various treatments. Journal of Agricultural and Food Chemistry. 2000;48:5962–5968. doi: 10.1021/jf000956t. [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR. Molecular characterization of the Arabidopsis 9- cis-epoxycarotenoid dioxygenase gene family. The Plant Journal. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. The Plant Journal. 2008;54:733–749. doi: 10.1111/j.1365-313X.2008.03447.x. [DOI] [PubMed] [Google Scholar]
- Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D. The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ϵ-ring hydroxylation activity. Proceedings of the National Academy of Sciences, USA. 2004;101:402–407. doi: 10.1073/pnas.2237237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, Maass D, Voegel T, DellaPenna D, Beyer P. Transcription factor RAP2.2 and its interacting partner SINAT2: stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiology. 2007;145:1073–1085. doi: 10.1104/pp.107.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ichimura K, Kanekatsu M, van Doorn WG. Gene expression in opening and senescing petals of morning glory (Ipomoea nil) flowers. Plant Cell Report. 2007;26:823–835. doi: 10.1007/s00299-006-0285-4. [DOI] [PubMed] [Google Scholar]
- Yamagishi M, Kishimoto S, Nakayama M. Carotenoid composition and changes in expression of carotenoid biosynthetic genes in tepals of Asiatic hybrid lily. Plant Breeding. 2009;128:172–177. [Google Scholar]
- Yu B, Lydiate DJ, Young LW, Schäfer UA, Hannoufa A. Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsolon cyclase. Transgenic Research. 2008;17:573–585. doi: 10.1007/s11248-007-9131-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.