Abstract
Kupffer cells are macrophages in the liver whose major role is to clear circulating pathogens. Decreased phagocytic capacity of Kupffer cells may result in severe systemic infection. We tested the hypothesis that the depressed Kupffer cell phagocytic capacity following trauma-hemorrhage is enhanced by estrogen administration and this occurs due to maintenance of Fc receptor expression and cellular ATP content via the activation of Akt. Male C3H/HeN mice were subjected to sham operation or trauma-hemorrhage and sacrificed 2 h thereafter. Estrogen, with or without an estrogen receptor antagonist (ICI 182,780), a PI3K inhibitor (Wortmannin), or vehicle, was injected during resuscitation. Kupffer cell phagocytic capacity was tested in vivo. The expression of Fc receptors, of Akt phosphorylation, of p38 MAPK phosphorylation, of DNA binding activity of NF-κB and ATP content of Kupffer cells were also determined. Trauma-hemorrhage suppressed Kupffer cell phagocytosis by decreasing Fc receptor expression and Akt activation; however, it induced p38 MAPK activation and increased NF-κB activity. Cellular ATP levels were also decreased following trauma-hemorrhage. Administration of estrogen following trauma-hemorrhage increased phospho-Akt levels and normalized all the parameters described as well as plasma levels of TNF-α, IL-6, and IL-10. Coadministration of ICI 182,780 or Wortmannin abolished the beneficial effects of estrogen in improving the phagocytic capacity of Kupffer cells following trauma-hemorrhage. Thus, activation of Akt plays a crucial role in mediating the salutary effect of estrogen in restoring trauma-hemorrhage-induced suppression of Kupffer cell phagocytosis.
The immune response to microbial pathogens relies on both innate and adaptive immunity. Macrophages, as specialized phagocytes, play an important role in bridging innate and adaptive immunity by killing the pathogens through the process of phagocytosis, and presenting the microbial peptides to T cells that result in specific T cell activation. Therefore, phagocytosis is one of the first steps of the host defense system to remove pathogens and trigger the adaptive immune response. Kupffer cells are the largest population of resident macrophages in the body and act as an important defense barrier against systemic pathogens (1). By i.v. injecting bacteria or colloid particles, studies have shown that Kupffer cells can take up 80–90% of all the injected bioparticles and play a dominant role in the clearance of circulating pathogens (2, 3). Trauma-hemorrhage is characterized by prolonged immune suppression and profound deterioration of immune functions leading to secondary complications such as sepsis, multiple organ failure and subsequent mortality (4–8). Previous studies have demonstrated that Kupffer cells can elicit a profound inflammatory response and their phagocytic capacity is depressed following trauma-hemorrhage (9, 10); however, the precise mechanism responsible for producing the depressed capacity following trauma-hemorrhage and the mechanisms regulating phagocytosis under those conditions are not known. In this regard, PI3K and its downstream signaling molecule, protein kinase B (also known as Akt), have been shown to play an important role in regulating phagocytosis. Although binding of IgG-opsonized particles to the Fc receptors triggers phagocytosis through actin polymerization, mobilization, membrane extension and particle engulfment (11), inhibition of PI3K/Akt blocks the Fc receptor-mediated phagocytosis of IgG-opsonized particles and bacteria (12). Furthermore, our previous studies have demonstrated that the activation of Akt (phospho-Akt) is decreased in the liver following trauma-hemorrhage (13). Thus, it is likely that a decrease in phospho-Akt may also influence the Kupffer cell phagocytic capacity following trauma-hemorrhage.
It has been demonstrated that estrogen modulates immune responsiveness, restores or normalizes the altered immune responses following trauma-hemorrhage (8, 14–18). Of numerous mechanisms that have been studied for the salutary effects of estrogen on immune function after trauma-hemorrhage, it has been consistently shown that the PI3K/Akt pathway mediates the protective effect of estrogen in vital organs such as heart, liver, and intestine (13, 19–22). In the current study, we tested the hypothesis that traumahemorrhage impairs Kupffer cell phagocytosis through inhibition of Akt activation, whereas administration of estrogen improves Kupffer cell phagocytosis by increasing Akt activation under those conditions.
Materials and Methods
Mouse trauma-hemorrhagic shock model
Male C3H/HeN mice 8-wk-old and weighing 22–25 g (Charles River Breeding Laboratories) were fasted overnight before the experiment but were allowed water ad libitum. All experiments were performed in adherence with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Animals were anesthetized with isoflurane (Minrad) and restrained in a supine position. A midline laparotomy (2 cm) was performed, which was then closed in two layers with sutures (Ethilon 6/0; Ethicon). Both femoral arteries and a femoral vein were cannulated with polyethylene-10 tubing (BD Biosciences). Blood pressure was measured via one of the arterial catheters using a blood pressure analyzer (MicroMed). Upon awakening, the mice were bled rapidly through the other arterial catheter to a mean arterial blood pressure of 35 ± 5 mm Hg within 10 min, which was then maintained for 90 min. At the end of that interval, the animals were resuscitated via the venous line with four times the shed blood volume using Ringer’s lactate solution over 30 min. After ligating the blood vessels, catheters were removed; the incisions were flushed with lidocaine and closed with sutures. Sham-operated animals underwent laparotomy and the same groin dissection, which included cannulation and ligation of the femoral artery and vein, but neither hemorrhage nor resuscitation was conducted. The animals were killed at 2 h after the end of resuscitation or sham operation.
Assignment of animal groups for treatment
Animals subjected to trauma-hemorrhage were allocated randomly into four groups receiving vehicle (cyclodextrin via i.v. administration; Sigma-Aldrich), 17β-estradiol (estrogen, 1 mg/kg body weight i.v.; Sigma-Aldrich), estrogen plus the PI3K inhibitor Wortmannin (1 mg/kg body weight i.p.; Sigma-Aldrich), or estrogen plus a high-affinity estrogen receptor antagonist ICI 182,780 (3 mg/kg body weight i.p.; Tocris Cookson) at the beginning of resuscitation. Sham animals received injection of either vehicle or estrogen at time points corresponding to the trauma-hemorrhage groups.
Preparation of plasma and Kupffer cells
Blood was obtained via cardiac puncture and was centrifuged at 2500 × g for 10 min. The plasma was collected and stored at −80°C until analyzed.
Kupffer cells were isolated as previously described (23). In brief, the portal vein was catheterized with a 27-gauge needle, and the liver was perfused with 20 ml of HBSS (Life Technologies) at 37°C, immediately followed by perfusion with 15 ml of 0.05% collagenase IV (Worthington Biochemical) in HBSS with 0.5 mM CaCl2 (Sigma-Aldrich) at 37°C. The liver was then removed and transferred to a petri dish containing the mentioned collagenase IV solution. The liver was minced, incubated for 15 min at 37°C, and passed through a sterile mesh stainless steel screen into a beaker containing 40 ml of cold HBSS with 10% FBS. The hepatocytes were removed by centrifugation at 50 × g for 3 min. The residual cell suspension was washed twice by centrifugation at 800 × g for 10 min at 4°C in HBSS. The cells were then resuspended in complete William’s E medium containing 10% FBS and antibiotics (50 U/ml penicillin, 50 µg/ml streptomycin, and 20 µg/ml gentamicin, all from Life Technologies) and layered over 16% Histodenz (Sigma-Aldrich) in HBSS and centrifuged at 3000 × g for 45 min at 4°C. After removing the nonparenchymal cells from the interface, the cells were washed twice by centrifugation (800 × g for 10 min at 4°C) in complete William’s E medium. Following this step, different procedures were used for different studies. For the determination of ATP content or for Kupffer cell culture, Kupffer cells were resuspended in complete RPMI 1640 medium at a concentration of 5 × 106/ml. For the measurement of phospho-Akt, the Kupffer cells were washed two times in PBS and lysed in the lysis buffer according to the manufacturer’s instructions (see below), then stored at −80°C until assayed. For the phagocytosis assay and the measurement of Fc receptor expression, the Kupffer cells were washed in PBS twice and resuspended in staining buffer (1% FBS and 0.09% sodium azide in PBS) followed by the steps described below for flow cytometric study.
Kupffer cell culture
The cells were resuspended in complete RPMI 1640 medium and plated in a 96-well plate at a cell density of 5 × 106 cells/ml. After 2 h of incubation (37°C, 95% humidity, and 5% CO2), nonadherent cells were removed by washing with complete RPMI 1640 medium. We compared the number of adherent cells at the end of 2 h and found no significant difference in the number of adherent cells from sham and trauma-hemorrhage mice. The cells were then cultured under the described conditions for 24 h with 1 µg/ml LPS (Sigma-Aldrich). The cell-free supernatants were harvested and stored at −80°C until assayed.
Preparation of phagocytosis assays
Because lysosomes are acidified to kill ingested pathogens following phagocytosis, a pHrodo Escherichia coli bioparticle conjugate (Molecular Probes), which emits strong fluorescence in acidic surroundings was used to evaluate Kupffer cell phagocytosis. For the in vivo phagocytosis assay, the particle was dissolved in 0.9% sodium chloride solution at a concentration of 2 mg/ml, and 400 µg/mouse was i.v. injected right before the beginning of resuscitation or at corresponding time points for sham in all the animals if not differently stated.
Measurement of phagocytosis and expression of Fc receptors by flow cytometry
The Kupffer cells were resuspended in staining buffer and incubated with FITC-conjugated anti-CD16/CD32 (FcγRIII/RII) Ab (clone 2.4G2; BD Pharmingen) for 15 min on ice. After washing the cells with staining buffer two times, they were incubated on ice with purified anti-CD16/CD32 Fc blocking Ab (BD Pharmingen) for another 15 min. In the presence of the blocking Ab, the cells were then incubated on ice with allophycocyanin-Alexa Fluor 750-conjugated anti-CD11b Ab for 45 min. Following two washes in staining buffer, the cells were resuspended in 200 µl of staining buffer and analyzed using the LSRII flow cytometer (BD Biosciences). Isotype-matched IgGs were used as a nonspecific staining control, and appropriate single-stained compensation controls were also used. The cell populations gated as CD11b+ were analyzed. Data analysis was conducted using the FACSDiva software (BD Biosciences). For the measurement of phagocytosis, cells were prepared the same as described but without the staining of FITC-conjugated anti-CD16/CD32 Ab.
Measurement of phospho-Akt and phospho-p38α by ELISA
The relative amount of phospho-Akt and phospho-p38α protein in Kupffer cells was determined using Duoset IC ELISA kit for phospho-Akt and phospho-p38α (R&D Systems). Briefly, lysis buffers were prepared according to the manufacturer’s instructions, and a 96-well microplate was coated with phospho-Akt or phospho-p38α capture Ab and incubated overnight at room temperature before use. Isolated Kupffer cells were solubilized in lysis buffer at a concentration of 1 × 107 cells/ml and the cell lysates were added into wells at a volume of 100 µl/well and incubated for 2 h. The phospho-Akt or phospho-p38α detection Ab was then added to each well after the washing procedure, followed by 1 h of incubation. The relative amount of phospho-Akt and phospho-p38α protein was then detected using a standard streptavidin-HRP format and the absorbance was read at 450 nm followed by 570 nm subtraction. The value of phospho-Akt and phospho-p38α was normalized to the protein content of each group before statistical analysis.
DNA binding activity of activated NF-κB
DNA binding activity was evaluated using the TransAM ELISA method (Active Motif) according to the manufacturer’s instructions. To prepare nuclear extract, 5 × 106 cells were washed with ice-cold PBS containing phosphatase inhibitors. Cells were resuspended in 500 µl of cytosolic lysis buffer, which was provided by the manufacturer. After 15 min, nuclei were separated by centrifugation at 14,000 × g for 30 s. The supernatant that contained the cytosolic proteins was then removed. The pellet, containing nuclei, was resuspended in 50 µl of lysis buffer provided by the TransAM ELISA kit. After 30 min of incubation, nuclei were clarified by high-speed centrifugation (14,000 × g for 10 min). Nuclear extract was assayed for the DNA binding activity of NF-κB according to the protocol of the TransAM kit. Briefly, the DNA binding motif of NF-κB (5′-GGGACTTTCC-3′) is coated to a 96-well plate. When nuclear extracts are added to the plate, the activated NF-κB binds to the DNA causing the exposure of an epitope, which is recognized by a primary Ab directed against p65. A HRP-conjugated secondary Ab provides a sensitive colorimetric reaction, which is quantified by spectrophotometry. Absorbances were read at 450 nm with a reference wavelength of 655 nm.
Measurement of Kupffer cell ATP content
Cellular ATP content of Kupffer cells was measured using an ATP determination kit (Molecular Probes). Briefly, a standard reaction solution containing luciferin, firefly luciferase, DTT, and reaction buffer was prepared according to the manufacturer’s instruction. For determination of ATP, 90 µl of the standard reaction solution was added to 10 µl of suspended Kupffer cells or ATP standard. The measurement was performed with a Victor 3V multilabel counter (PerkinElmer Precisely).
Flow cytometric analysis of cytokine concentration
Plasma and Kupffer cell supernatant cytokine concentrations were determined with cytometric bead arrays using flow cytometry according to the manufacturer’s instructions (BD Pharmingen). Briefly, 50 µl of mixed capture beads was incubated with a 50-µl sample for 1 h at 25°C, and then 50 µl of mixed PE-conjugated detection Ab was added. After incubation for 1 h at 25°C in the dark, the complexes were washed twice and analyzed using the LSRII flow cytometer. Data analysis was conducted using the FACSDiva and FCAP array software (BD Biosciences).
Statistics
Statistical analysis was performed using Sigma-Stat computer software (SPSS). Statistical significance was assumed where probability values of less than 0.05 were obtained. Comparisons between groups were performed using one-way ANOVA followed by Tukey’s test. Results are expressed as mean ± SEM.
Results
Akt, phospho-38α phosphorylation, NF-κB activity and phagocytic capacity in Kupffer cells following trauma-hemorrhage
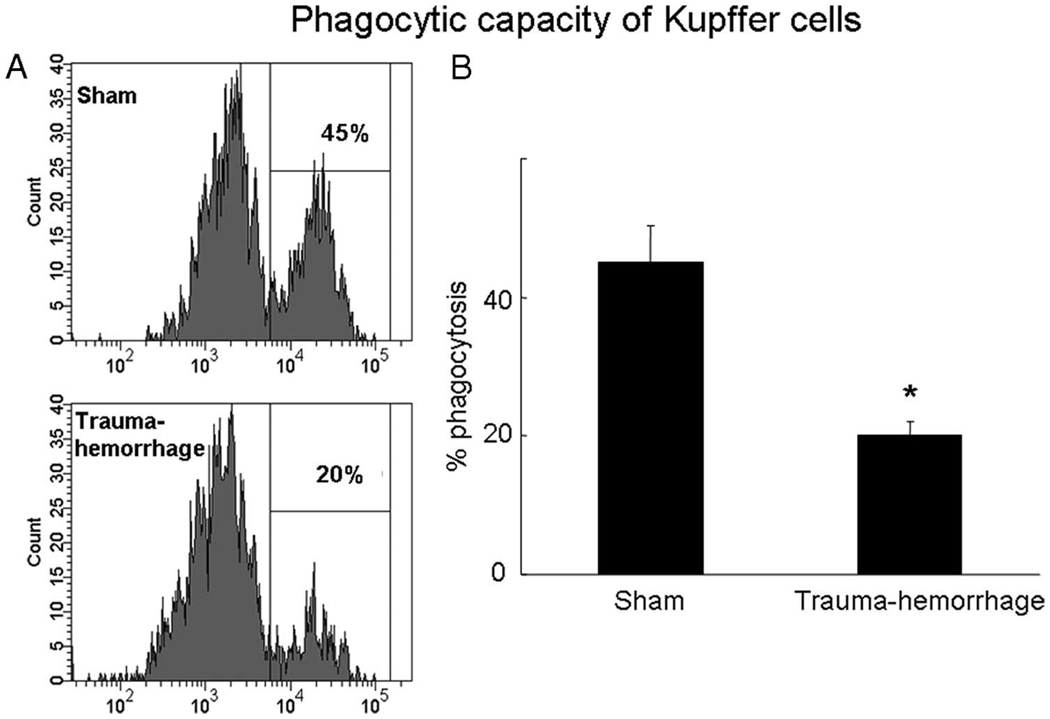
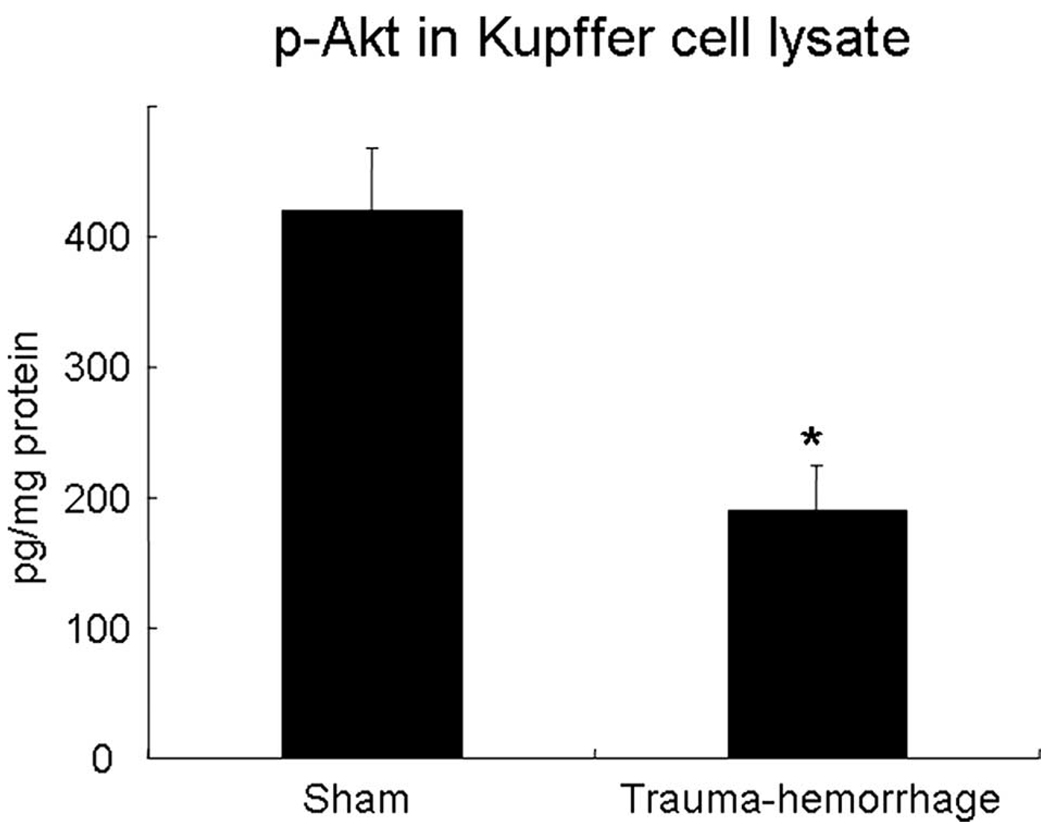
Fig. 1 shows those Kupffer cells that have ingested bioparticles. Kupffer cell phagocytosis was suppressed by more than 50% following trauma-hemorrhage compared with shams. In parallel, the phosphorylation of Akt was also decreased by ~50% following trauma-hemorrhage compared with shams (Fig. 2).
FIGURE 1.
Kupffer cell phagocytic capacity following trauma-hemorrhage. Mice were subjected to sham operation or trauma-hemorrhage. In vivo phagocytosis assays were performed by injection of E. coli bioparticles at the beginning of resuscitation. Kupffer cells were harvested 2 h after resuscitation and analyzed by flow cytometry as described in Materials and Methods. A, The gated region and percentage shown in the representative histogram indicates the percentage of Kupffer cells that had ingested bioparticles. B, The percentage of phagocytosis in sham and trauma-hemorrhage groups. Data are shown as mean ± SE for n = 6 animals/ group. *, p < 0.05 compared with sham.
FIGURE 2.
Akt phosphorylation in Kupffer cells following trauma-hemorrhage. Mice were subjected to sham operation or trauma-hemorrhage. E. coli bioparticle conjugates for the phagocytosis assay were injected into each group immediately before resuscitation. Livers were harvested aseptically 2 h after resuscitation. Kupffer cells were lysed immediately after they were isolated and purified, and the concentrations of phospho-Akt in the cell lysates were determined by a commercially available ELISA kit. Data are normalized to protein content and are shown as mean ± SE for n = 6 animals/group. *, p < 0.05 compared with sham.
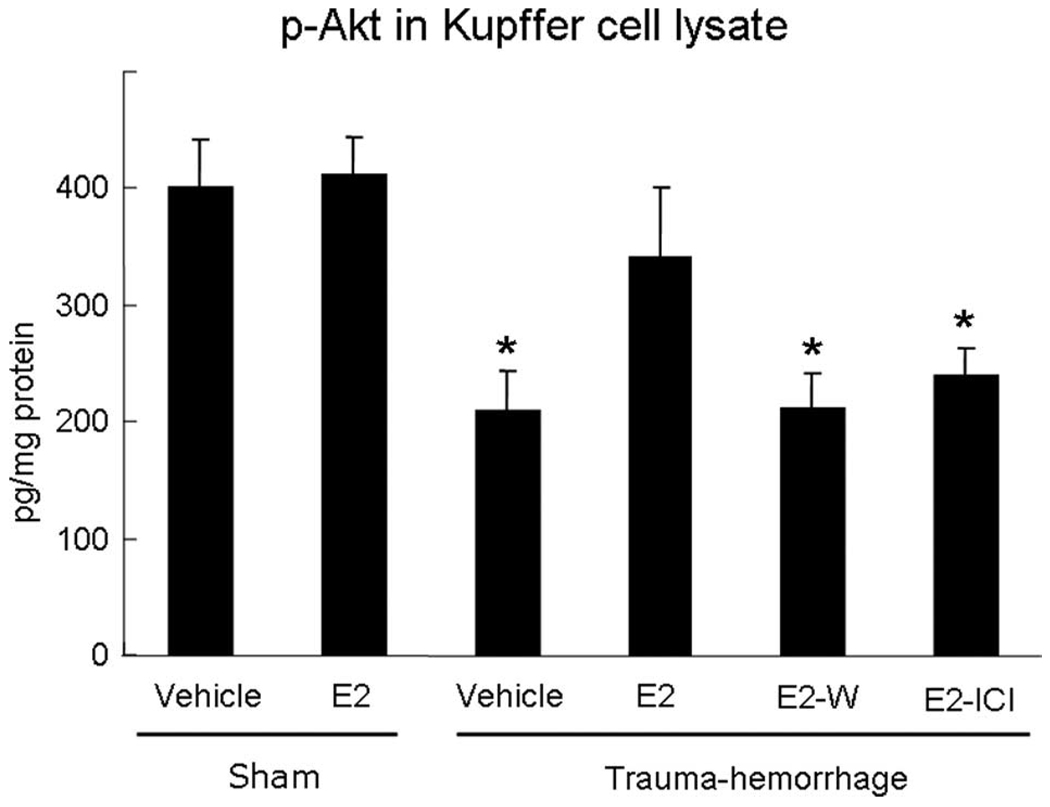
We next examined whether estrogen administration could prevent the decrease in phospho-Akt following trauma-hemorrhage. Similar to results shown in Fig. 2, trauma-hemorrhage induced a marked decrease in Akt phosphorylation compared with shams. Administration of estrogen prevented the decrease in Akt phosphorylation following trauma-hemorrhage. The estrogen-mediated restoration of Akt phosphorylation following trauma-hemorrhage was abolished when Wortmannin was coadministered with estrogen. Furthermore, coadministration of ICI 182,780 with estrogen also prevented the estrogen-induced increase in Akt phosphorylation. There was no difference in Akt phosphorylation in sham animals treated with estrogen or vehicle (Fig. 3).
FIGURE 3.
Akt phosphorylation in Kupffer cells. Mice were subjected to sham operation or trauma-hemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-ICI) immediately before resuscitation. Kupffer cells were lysed immediately after they were isolated and purified, and the concentrations of phospho-Akt in the cell lysates were determined by a commercially available ELISA kit as in Fig. 2 and as described in Materials and Methods. Data are normalized to protein content and shown as mean ± SE for n = 6 animals/group.*, p < 0.05 compared with sham and estrogen-treated groups.
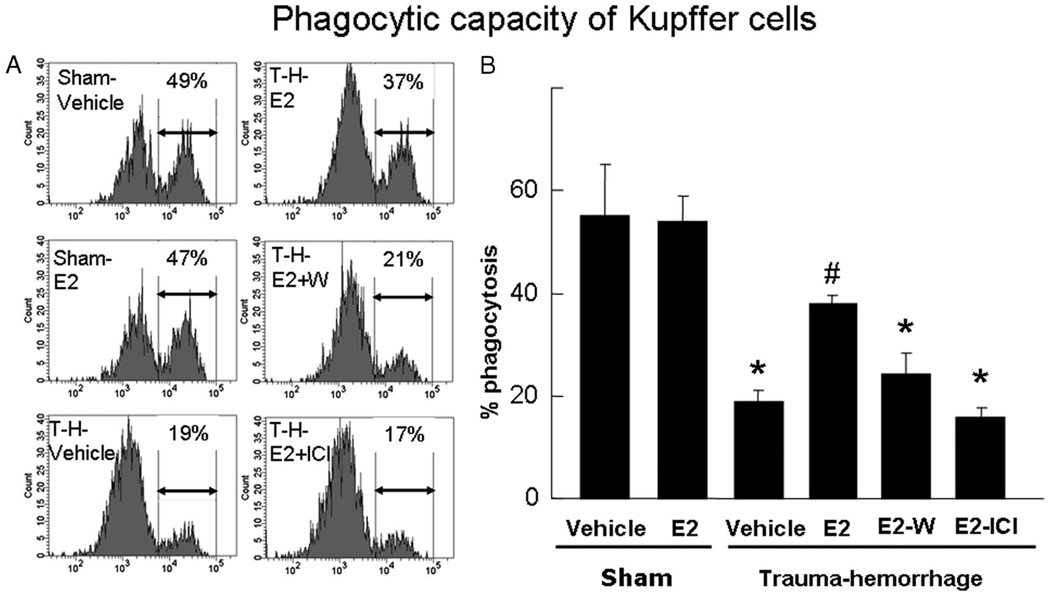
Administration of estrogen also prevented the decrease in Kupffer cell phagocytic capacity following trauma-hemorrhage (Fig. 4). The enhancement in the phagocytic capacity by estrogen following trauma-hemorrhage was abolished when either Wortmannin or ICI 182,780 was coadministered with estrogen. Administration of estrogen in sham animals did not influence the Kupffer cell phagocytic capacity (Fig. 4).
FIGURE 4.
Phagocytic capacity of Kupffer cells. Mice were subjected to sham operation or trauma-hemorrhage (T-H) and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-ICI) immediately before resuscitation. E. coli bioparticle conjugates for the phagocytosis assay were injected into each group immediately before resuscitation. Livers were harvested aseptically 2 h after resuscitation, and Kupffer cells were isolated. The Kupffer cells were surface stained with an allophycocyanin-Alexa Fluor 750-conjugated CD11b Ab as described in Materials and Methods and were analyzed by flow cytometry. The percentage of Kupffer cells that ingested bioparticles is shown by representative histograms (A) and as mean ± SE (B) for n = 6 animals/group. *, p < 0.05 compared with both sham groups; #, p < 0.05 compared with the other three trauma-hemorrhage groups.
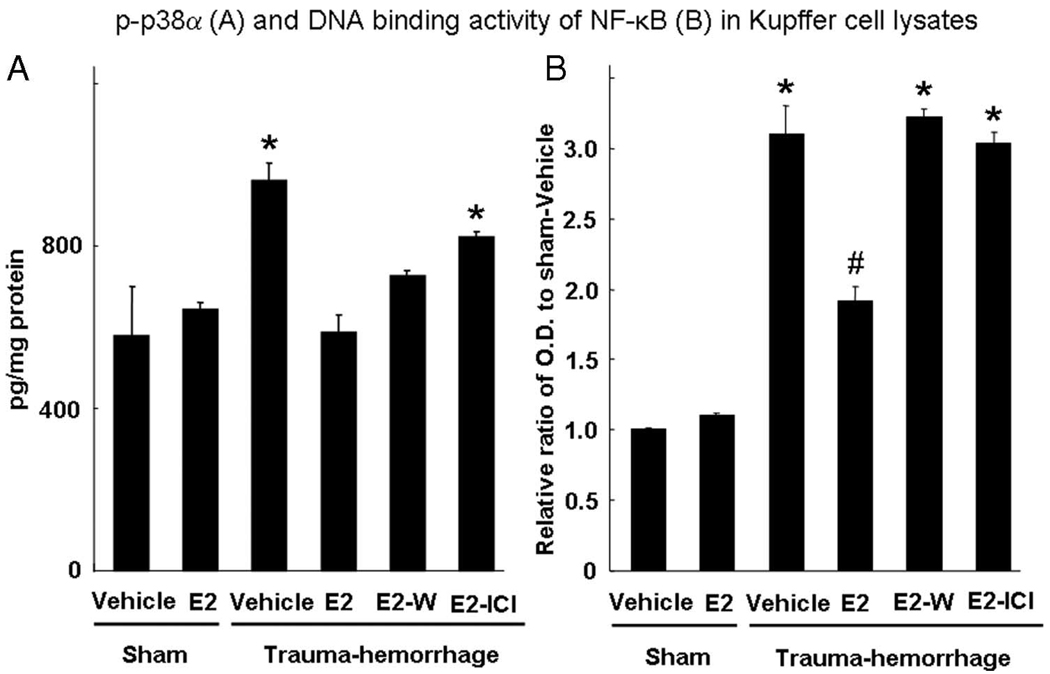
To determine whether there were any other important signaling molecules that might mediate the salutary effect of estrogen on Kupffer cell phagocytosis following trauma-hemorrhage, we studied the activation of p38α MAPK and NF-κB of Kupffer cells under those conditions. In our previous studies, these two molecules have been shown to play an important role in the activation of Kupffer cell proinflammatory response following trauma-hemorrhage (10, 24–26). Unlike those seen in Akt activation, trauma-hemorrhage induced a marked increase in p38α phosphorylation compared with shams. Administration of estrogen prevented the increase in p38α phosphorylation following trauma-hemorrhage. The estrogen-mediated restoration of p38α phosphorylation following trauma-hemorrhage was abolished when ICI 182,780 was coadministered with estrogen. There was no difference in p38α phosphorylation in sham animals treated with estrogen or vehicle (Fig. 5A).
FIGURE 5.
p38α phosphorylation (A) and DNA binding activity of NF-κB (B) in Kupffer cells. Mice were subjected to sham operation or traumahemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-ICI) immediately before resuscitation. Kupffer cells were lysed immediately after they were isolated and purified, and the concentration of phospho-p38α in the cell lysates was determined by a commercially available ELISA kit. Nuclear extracts of Kupffer cells were prepared as described in Materials and Methods for measuring DNA binding activity of NF-κB. Data are normalized to protein content and shown as mean ± SE for n = 6 animals/group.*, p < 0.05 compared with the other groups; #, p < 0.05 compared with sham groups.
Trauma-hemorrhage also induced a significant increase in NF-κB activity compared with shams. Administration of estrogen suppressed the increase in NF-κB activity following trauma-hemorrhage. The estrogen-mediated suppression of NF-κB activity following trauma-hemorrhage was abolished when Wortmannin or ICI 182,780 was coadministered with estrogen. There was no difference in NF-κB activity in sham animals treated with estrogen or vehicle (Fig. 5B).
CD16/32 (FcγRIII/RII) expression in Kupffer cells
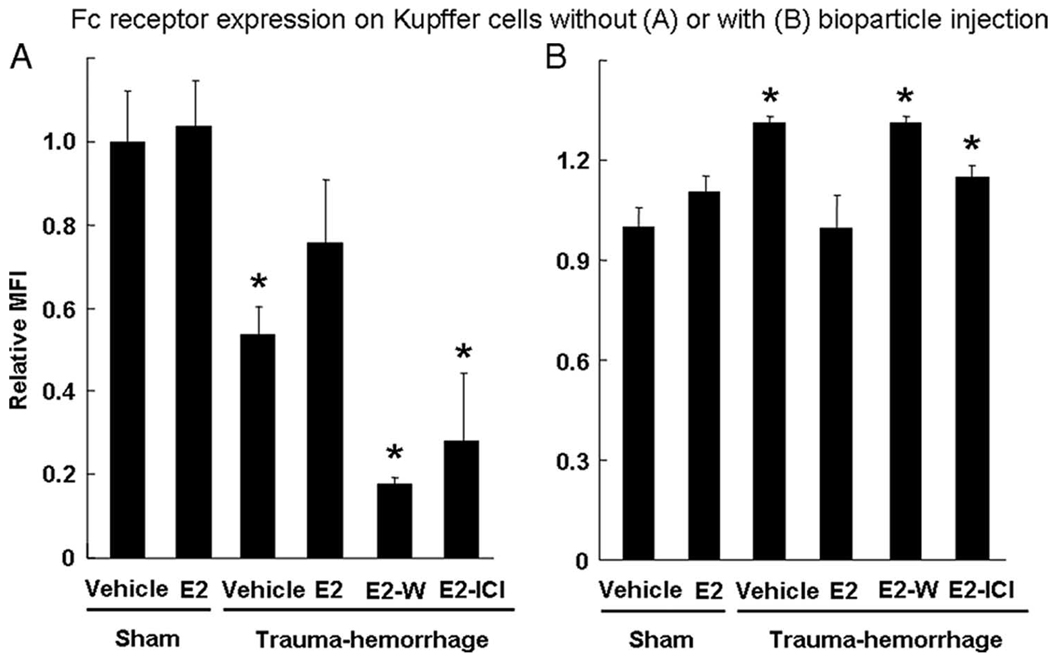
To determine whether there were any changes in Fc receptor expression following trauma-hemorrhage and whether such changes contribute to the altered phagocytic capacity of Kupffer cells, Kupffer cells were surface-stained with FITC-conjugated anti-CD16/32 after isolation and were analyzed by flow cytometry. The relative mean fluorescence intensity of each group was compared with the sham vehicle group. In two separate experiments, mice were either injected or not with pHrodo E. coli bioparticles. When bioparticles were not injected, the Fc receptor expression on the Kupffer cells from mice following trauma-hemorrhage was significantly lower than that in sham group (Fig. 6A). Furthermore, administration of estrogen significantly enhanced the expression of Fc receptor following trauma-hemorrhage and normalized it to sham levels. The effects of estrogen were abolished when either Wortmannin or ICI 182,780 was coadministered with estrogen. The expression of Fc receptors from these two groups was similar to those of cells treated with vehicle after trauma-hemorrhage. No effect of estrogen on Fc receptor expression was observed in shams (Fig. 6A).
FIGURE 6.
Fc receptor expression on Kupffer cells without (A) or with injection of E. coli bioparticle conjugates (B). Mice were subjected to sham operation or trauma-hemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-I) immediately before resuscitation. For all the groups in B, E. coli bioparticle conjugates for the phagocytosis assay were injected into each group immediately before resuscitation. Livers were harvested aseptically 2 h after resuscitation and Kupffer cells were isolated. The Kupffer cells were surface stained with FITC-conjugated CD16/32 Ab and allophycocyanin-Alexa Fluor 750-conjugated CD11b Ab as described in Materials and Methods and were analyzed by flow cytometry. The mean fluorescence intensity was normalized to sham-vehicle group. Data are shown as mean ± SE for n = 6 animals/group. *, p < 0.05 compared with sham and estrogen-treated group.
When bioparticles were injected, the Fc receptor expression on the Kupffer cells showed a reverse pattern compared with the cells from mice not injected with bioparticles. The results summarized in Fig. 6B indicate a higher Fc receptor expression on Kupffer cells following trauma-hemorrhage comparison of shams. However, cells from mice treated with estrogen following trauma-hemorrhage had a similar level of Fc receptor expression as shams. The effect of estrogen on Fc receptor expression was abolished when Wortmannin or ICI 182,780 was coadministered with estrogen.
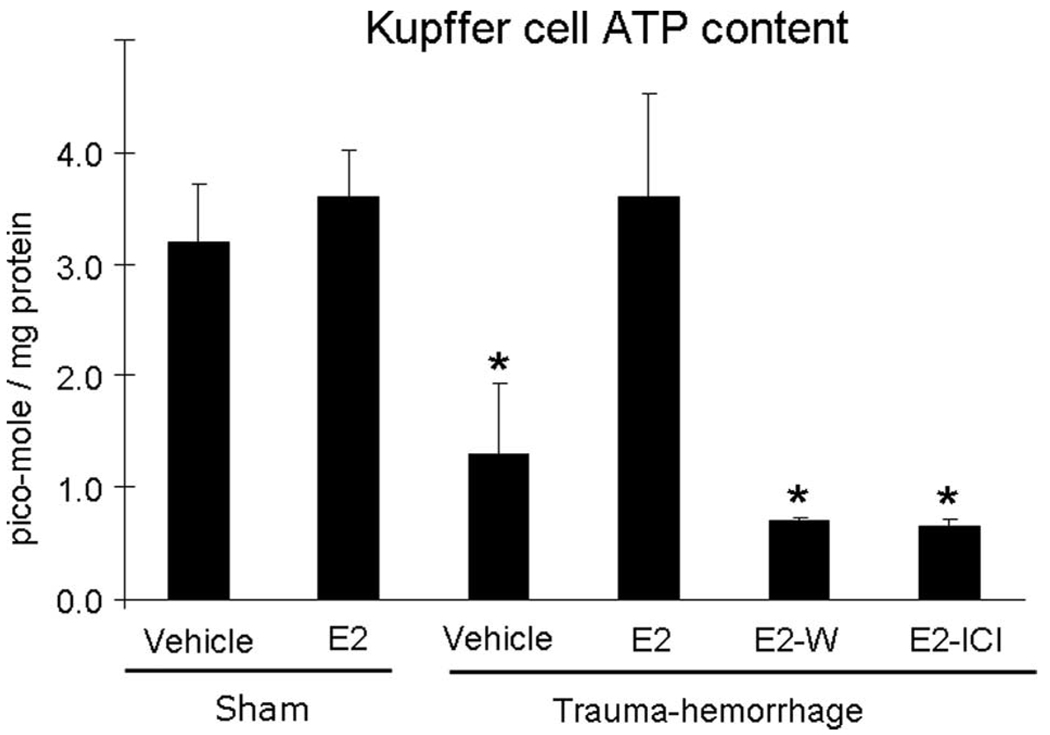
Kupffer cell ATP content
ATP levels of Kupffer cells from mice treated with vehicle following trauma-hemorrhage were significantly decreased compared with those of sham mice (Fig. 7). Administration of estrogen restored Kupffer cell ATP levels, which were abolished by the coadministration of Wortmannin or ICI 182,780. The ATP levels of the cells from sham mice treated with or without estrogen showed no significant difference (Fig. 7).
FIGURE 7.
ATP content in Kupffer cells. Mice were subjected to sham operation or trauma-hemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-I) immediately before resuscitation. E. coli bioparticle conjugates for the phagocytosis assay were injected into each group immediately before resuscitation. The livers were harvested aseptically 2 h after resuscitation and Kupffer cells were isolated. ATP contents were measured according to the manufacturer’s instructions for an ATP determination kit as described in Materials and Methods. Cell suspensions were mixed with a standard reaction solution and analyzed with luminometry. Data are shown as mean ± SE for n = 6 animals/group. *, p < 0.05 compared with sham and estrogen-treated group.
Plasma cytokine levels and Kupffer cell cytokine production capacity
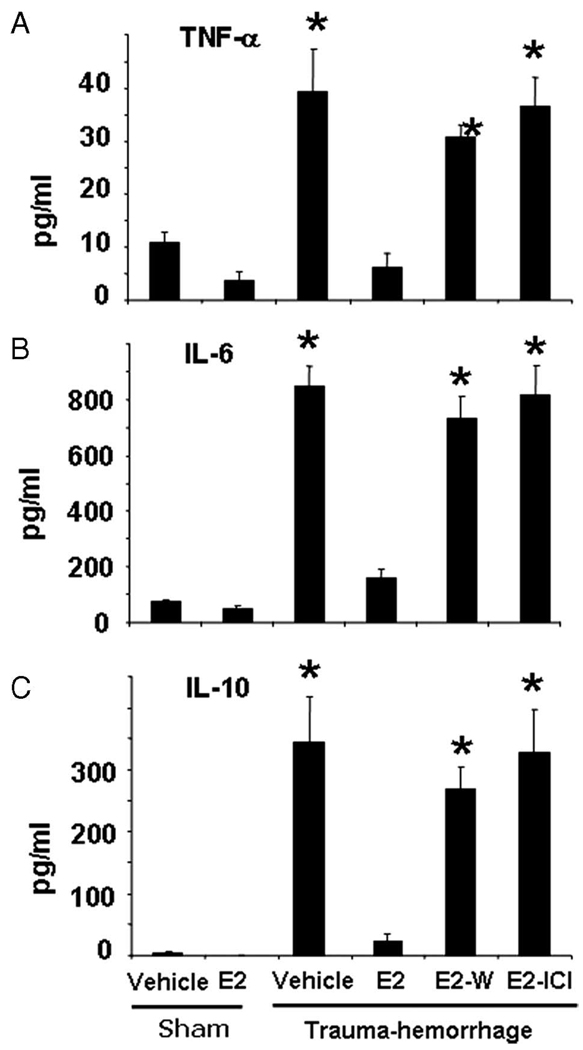
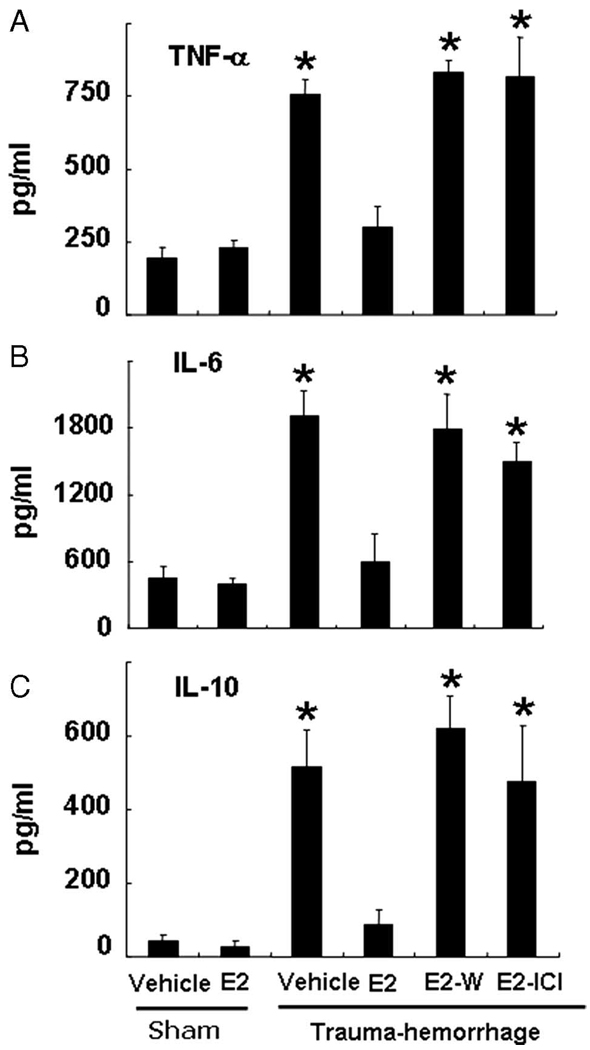
Trauma-hemorrhage induced a significant increase in the concentrations of TNF-α, IL-6 and IL-10 compared with those of shams (Fig. 8). Administration of estrogen following trauma-hemorrhage normalized plasma cytokine concentrations to sham levels (Fig. 8). The salutary effect of estrogen was abolished when Wortmannin or ICI 182,780 was coadministered with estrogen. The plasma cytokine concentrations in sham mice treated with or without estrogen showed no significant difference (Fig. 8).
FIGURE 8.
Plasma concentrations of TNF-α (A), IL-6 (B), and IL-10 (C). Mice were subjected to sham operation or trauma-hemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin, or estrogen plus ICI 182,780 (E2-I) before resuscitation. E. coli bioparticle conjugates for the phagocytosis assay were also injected into each group immediately before resuscitation. Blood was obtained 2 h after resuscitation or sham operation via cardiac puncture and was centrifuged. The plasma was collected and stored at −80°C until analyzed. Plasma cytokine concentrations were determined using the cytometric bead array technique described in Materials and Methods. Data are shown as mean ± SE for n = 6 animals/group. *, p < 0.05 compared with sham and estrogen-treated group.
The levels of TNF-α, IL-6, and IL-10 were also measured in Kupffer cell culture supernatants. The results showed that Kupffer cell production capacity of all three cytokines was significantly increased following trauma-hemorrhage compared with shams (Fig. 9). Administration of estrogen following trauma-hemorrhage normalized Kupffer cell cytokine production. However, the salutary effects of estrogen were abolished when Wortmannin or ICI 182,780 was coadministered with estrogen. Administration of estrogen in sham mice did not significantly affect Kupffer cell cytokine production (Fig. 9).
FIGURE 9.
Kupffer cell cytokine production capacity of TNF-α (A), IL-6 (B), and IL-10 (C). Mice were subjected to sham operation or trauma-hemorrhage and were treated with vehicle, estrogen (E2), estrogen plus Wortmannin (E2-W), or estrogen plus ICI 182,780 (E2-I) before resuscitation. E. coli bioparticle conjugates for the phagocytosis assay were also injected into each group right before resuscitation. The Kupffer cells were harvested as described in Materials and Methods. The cells were cultured in a 96-well plate at a cell density of 5 × 106 cells/ml for 24 h with 1 µg/ml LPS. The cell-free supernatants were harvested and stored at −80°C until assayed. Data are shown as mean ± SE for n = 6 animals/group. *, p < 0.05 compared with sham and estrogen-treated group.
Discussion
The immune response to microbial pathogens relies on both innate and adaptive immunity. Macrophages can kill the pathogens by phagocytosis and subsequently present Ag to T cells, resulting in T cell activation and initiation of the adaptive immune response. Phagocytosis, therefore, is the first step of the host defense system to remove pathogens. Because Kupffer cells are the largest population of resident macrophages in the body and act as an important defense barrier against systemic infection (2, 3), decreased phagocytic capacity of Kupffer cells may weaken both innate and adaptive immunity.
Previous studies from our laboratory have shown that Kupffer cells play an important role in the inflammatory response following trauma-hemorrhage (27). We have shown that following trauma-hemorrhage, Kupffer cells produce increased amounts of proinflammatory cytokines (i.e., IL-6, TNF-α) both in vitro and in vivo (27, 28). We also found that TLR4 up-regulation produced an inflammatory cascade involving activation of p38 MAPK and NF-κB, which in turn led to the release of proinflammatory cytokines (25, 29). In contrast to the increased Kupffer cell cytokine production following trauma-hemorrhage, the present study showed that the phagocytic capacity of the Kupffer cell was decreased. Hence, mechanisms independent from cytokine production may be responsible for the differences between phagocytosis and cytokine production in Kupffer cells following trauma-hemorrhage. Our results revealed that the expression of Fc receptors is significantly decreased on Kupffer cells following trauma-hemorrhage compared with shams. These findings are consistent with our previous reports indicating that the expression of Fc receptors on peritoneal macrophages as well as the Fc receptor-mediated phagocytosis of those cells was significantly decreased at 12 or 24 h after hemorrhage compared with controls (30, 31). We also measured the Fc receptor expression in animals that were injected with bioparticles. The patterns of the relative amount of Fc receptor expression between sham and trauma-hemorrhage groups were reversed compared with those not injected with bioparticles. Under these conditions, we found that the Fc receptor expression on Kupffer cells from sham mice was significantly lower than that in trauma-hemorrhage mice. This suggests that the Fc receptors were internalized along with the bioparticles during phagocytosis; hence the density of Fc receptors on the surface of Kupffer cells from sham groups was temporarily lower than that from the trauma-hemorrhage group.
Although numerous proteins and pathways are involved in the signal transduction during Fc receptor-mediated phagocytosis, the PI3K/Akt pathway is required for actin filament remodeling and membrane extension, a key process of phagocytosis (32–34). Studies have shown that Fc receptor stimulation can induce Akt activation. Furthermore, administration of the PI3K inhibitor Wortmannin abolished the Akt activation-induced phagocytosis (12, 35). Conversely, overexpression of Akt in macrophages enhances phagocytic capacity (36). Previous studies from our laboratory have also shown that Akt activation was decreased in various tissues and cell types (i.e., hepatocytes, cardiomyocytes, and intestine) following trauma-hemorrhage (13, 19, 21, 37). Therefore, it could be suggested that inhibition of Akt activation following trauma-hemorrhage is the key factor affecting numerous cellular processes including Kupffer cell phagocytic capacity.
It is well recognized that phagocytosis is an energy-consuming process. Borregaard and Herlin (38) compared ATP contents of resting and phagocytozing human neutrophils. They found that the ATP content fell rapidly during phagocytosis, indicating significant energy is needed for the phagocytic process. Moreover, to show that oxygen is required for ATP production, Leeper-Woodford and Mills (39) demonstrated that the ATP levels and phagocytic function of alveolar macrophages were decreased if the cells were cultured in hypoxic environment. Additionally, our recent studies showed that the ATP production is decreased in Kupffer cells following trauma-hemorrhage (40). In that study, we found that TLR4 expression in Kupffer cells from wild-type mice was increased 2 h after trauma-hemorrhage, whereas the ATP levels were decreased. In contrast, decreased ATP levels were not observed in TLR4-mutant mice (40). Akt also regulates the availability of ATP for the cells because activation of Akt maintains cell survival by allowing cells to continuously import glucose and prevent cells from ATP depletion (20, 41). Because regional hypoxia occurs following trauma-hemorrhage (42, 43), the traumahemorrhage-induced hypoxia produces an unfavorable environment for the production of ATP. Furthermore, the decreased phospho-Akt may exacerbate the ATP depletion, thus resulting in impaired phagocytosis following trauma-hemorrhage.
A number of our studies have shown that immune functions are depressed in males as well as in ovariectomized and aged females following trauma-hemorrhage, but are maintained in proestrus females under those conditions (8, 16, 17, 44). Our current results are in line with the previous studies demonstrating that decreased Kupffer cell phagocytic activity following trauma-hemorrhage is restored by estrogen administration. Our results indicate that the salutary effects of estrogen appear to be due to: 1) the improvement of Fc receptor expression; 2) the increased Akt activity; or 3) the increased ATP content. There are several studies supporting our findings. Gomez et al. (45) treated splenic macrophages with estrogens and found that estrogens enhance the clearance of IgG-sensitized cells by improving FcγR expression. Our previous studies demonstrated that the PI3K/Akt pathway mediates the cardioprotective and hepatoprotective effects of estrogen following trauma-hemorrhage, as coadministration of PI3K inhibitor abolished the beneficial effect of estrogen (13, 22). Furthermore, consistent with this study, we have also shown significantly lower ATP contents in Kupffer cells following trauma-hemorrhage compared with sham. These levels were, however, restored to sham levels by estrogen administration following trauma-hemorrhage (40). Our previous studies using selective estrogen receptor-α and estrogen receptor-β agonists have shown that the salutary effects of estrogen such as normalizing cellular responses following trauma-hemorrhage were mediated predominately via estrogen receptor-α (26, 46).
In contrast to suppressed phagocytic capacity following trauma-hemorrhage, the Kupffer cells are activated in terms of inflammatory cytokine production and are well known to be a major contributor of the increased plasma inflammatory cytokine levels following trauma-hemorrhage (27). The plasma inflammatory cytokine levels as well as Kupffer cell cytokine productive capacity were significantly increased after trauma-hemorrhage. Administration of estrogen normalized the cytokine levels to those of shams. This reciprocal regulation of decreased phagocytic capacity and increased cytokine production in Kupffer cells following trauma-hemorrhage suggests that different signaling pathways might be involved under these conditions. Our previous studies have shown that in addition to Akt, p38 MAPK is another important signaling molecule involved in the regulation of Kupffer cell activity following trauma-hemorrhage (10, 25). In contrast to the decreased activity of Akt, the current study and our previous studies revealed that the activity of the p38 MAPK in Kupffer cells was markedly increased following trauma-hemorrhage. Moreover, the activity of NF-κB of the Kupffer cells was also up-regulated following trauma-hemorrhage. Although NF-κB has been shown to be a downstream molecule of the PI3K/Akt or MAPK pathway and could be activated either via p38 MAPK or Akt activation (47), our results suggested that the increased activation of NF-κB following trauma-hemorrhage, was mediated by the increased p38 MAPK activation but not by Akt activation (10, 21, 22, 25). Furthermore, the increased NF-κB activity resulted in increased proinflammatory cytokine production by Kupffer cells following trauma-hemorrhage and was normalized by estrogen administration following trauma-hemorrhage. Our results also indicate that following trauma-hemorrhage, the phenotype of Kupffer cells changed from microbial-cleaning phagocytes into cytokine-producing cells. This change in phenotype may be considered a form of immune dysregulation, although estrogen was able to normalize it.
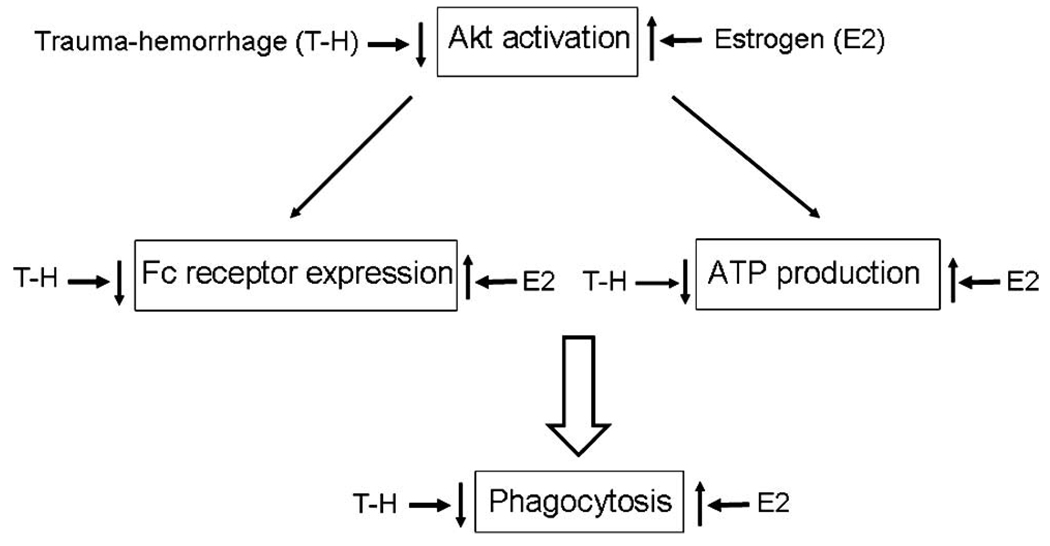
In summary, trauma-hemorrhage had a negative impact on the phagocytic capacity of Kupffer cells by inhibiting several critical mechanisms simultaneously. Furthermore, down-regulation of Akt activation worsened the ATP availability and Fc receptor-mediated phagocytosis, indicating the importance of Akt activation in phagocytic capacity. Administration of estrogen significantly increased phospho-Akt levels and restored Kupffer cell phagocytic capacity. Taken together, these show that activation of Akt plays an important role in mediating the salutary effect of estrogen in restoring Kupffer cell phagocytic capacity following trauma-hemorrhage (Fig. 10).
FIGURE 10.
Postulated diagrammatic presentation of estrogen (E2)-mediated restoration of Kupffer cell phagocytic capacity following trauma-hemorrhage.
Acknowledgments
We thank Bobbi Smith for skill and assistance in preparing this manuscript.
Footnotes
This work was supported by Grant R01 GM37127 from the National Institutes of Health (to I.H.C.).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Armbrust T, Ramadori G. Functional characterization of two different Kupffer cell populations of normal rat liver. J. Hepatol. 1996;25:518–528. doi: 10.1016/s0168-8278(96)80212-1. [DOI] [PubMed] [Google Scholar]
- 2.Ruiter DJ, van der MJ, Brouwer A, Hummel MJ, Mauw BJ, van der Ploeg JC, Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab. Invest. 1981;45:38–45. [PubMed] [Google Scholar]
- 3.Benacerraf B, Sebestyen MM, Schlossman S. A quantitative study of the kinetics of blood clearance of p32-labelled Escherichia coli and staphylococci by the reticuloendothelial system. J. Exp. Med. 1959;110:27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonk AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J. Immunol. 2008;180:2450–2458. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- 5.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28:684–693. doi: 10.1097/shk.0b013e31805362ed. [DOI] [PubMed] [Google Scholar]
- 6.Frankel DA, Acosta JA, Anjaria DJ, Porcides RD, Wolf PL, Coimbra R, Hoyt DB. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J. Surg. Res. 2007;143:276–280. doi: 10.1016/j.jss.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum DR. G-protein-coupled receptor 30 mediates estrogen’s nongenomic effects after hemorrhagic shock and trauma. Am. J. Pathol. 2007;170:1148–1151. doi: 10.2353/ajpath.2007.070025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(Suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 9.Frink M, Hsieh YC, Hu S, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of salutary effects of finasteride on post-traumatic immune/inflammatory response: upregulation of estradiol synthesis. Ann. Surg. 2007;246:836–843. doi: 10.1097/SLA.0b013e318158fca0. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Estrogen-mediated activation of non-genomic pathway improves macrophages cytokine production following trauma-hemorrhage. J. Cell Physiol. 2008;214:662–672. doi: 10.1002/jcp.21255. [DOI] [PubMed] [Google Scholar]
- 11.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 12.Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fcγ receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed] [Google Scholar]
- 13.Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 upregulation. J. Leukocyte Biol. 2007;82:1019–1026. doi: 10.1189/jlb.0607355. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Yu HP, Suzuki T, Szalay L, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. The role of estrogen receptor subtypes in ameliorating hepatic injury following trauma-hemorrhage. J. Hepatol. 2007;46:1047–1054. doi: 10.1016/j.jhep.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu HP, Yang S, Choudhry MA, Hsieh YC, Bland KI, Chaudry IH. Mechanism responsible for the salutary effects of flutamide on cardiac performance after trauma-hemorrhagic shock: upregulation of cardiomyocyte estrogen receptors. Surgery. 2005;138:85–92. doi: 10.1016/j.surg.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J. Immunol. 2006;177:4514–4520. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki T, Choudhry MA, Suzuki T, Schwacha MG, Bland KI, Chaudry IH. 17β-estradiol’s salutary effects on splenic dendritic cell functions following trauma-hemorrhage are mediated via estrogen receptor-α. Mol. Immunol. 2008;45:376–385. doi: 10.1016/j.molimm.2007.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu JT, Kan WH, Hsieh YC, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of estrogen-mediated improvement in cardiac function after trauma-hemorrhage: p38-dependent normalization of cardiac Akt phosphorylation and glycogen levels. Shock. 2008;30:372–378. doi: 10.1097/SHK.0b013e318164f25c. [DOI] [PubMed] [Google Scholar]
- 20.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J. Leukocyte Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- 22.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann. Surg. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Chaudry IH. The role of MIP-1α in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J. Immunol. 2008;181:2806–2812. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 24.Thobe BM, Frink M, Hildebrand F, Schwacha MG, Hubbard WJ, Choudhry MA, Chaudry IH. The role of MAPK in Kupffer cell Toll-like receptor (TLR) 2-, TLR4-, and TLR9-mediated signaling following trauma-hemorrhage. J. Cell Physiol. 2007;210:667–675. doi: 10.1002/jcp.20860. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh YC, Frink M, Thobe BM, Hsu JT, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. 17Beta-estradiol down-regulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Mol. Immunol. 2007;44:2165–2172. doi: 10.1016/j.molimm.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. 17β-estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominately via estrogen receptor-α. Surgery. 2006;140:141–148. doi: 10.1016/j.surg.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, Chaudry IH. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am. J. Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol. Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Thobe BM, Frink M, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Src family kinases regulate p38 MAPK-mediated IL-6 production in Kupffer cells following hypoxia. Am. J. Physiol. 2006;291:C476–C482. doi: 10.1152/ajpcell.00076.2006. [DOI] [PubMed] [Google Scholar]
- 30.Ayala A, Perrin MM, Wagner MA, Chaudry IH. Enhanced susceptibility to sepsis after simple hemorrhage: depression of Fc and C3b receptor-mediated phagocytosis. Arch. Surg. 1990;125:70–74. doi: 10.1001/archsurg.1990.01410130076010. [DOI] [PubMed] [Google Scholar]
- 31.Rana MW, Ayala A, Dean RE, Chaudry IH. Decreased Fc receptor expression on macrophages following simple hemorrhage as observed by scanning immunoelectron microscopy. J. Leukocyte Biol. 1990;48:512–518. doi: 10.1002/jlb.48.6.512. [DOI] [PubMed] [Google Scholar]
- 32.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian Y, Corum L, Meng Q, Blenis J, Zheng JZ, Shi X, Flynn DC, Jiang BH. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am. J. Physiol. 2004;286:C153–C163. doi: 10.1152/ajpcell.00142.2003. [DOI] [PubMed] [Google Scholar]
- 34.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 35.Tilton B, Andjelkovic M, Didichenko SA, Hemmings BA, Thelen M. G-Protein-coupled receptors and Fcγ-receptors mediate activation of Akt/protein kinase B in human phagocytes. J. Biol. Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 36.Ganesan LP, Wei G, Pengal RA, Moldovan L, Moldovan N, Ostrowski MC, Tridandapani S. The serine/threonine kinase Akt Promotes Fcγ receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J. Biol. Chem. 2004;279:54416–54425. doi: 10.1074/jbc.M408188200. [DOI] [PubMed] [Google Scholar]
- 37.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 38.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leeper-Woodford SK, Mills JW. Phagocytosis and ATP levels in alveolar macrophages during acute hypoxia. Am. J. Respir. Cell Mol. Biol. 1992;6:326–334. doi: 10.1165/ajrcmb/6.3.326. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YC, Frink M, Kawasaki T, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Downregulation of TLR4-dependent ATP production is critical for estrogen-mediated immunoprotection in Kupffer cells following trauma-hemorrhage. J. Cell Physiol. 2007;211:364–370. doi: 10.1002/jcp.20943. [DOI] [PubMed] [Google Scholar]
- 41.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson Y, Hostmann A, Matenov A, Ertel W, Oberholzer A. Erythropoiesis in multiply injured patients. J. Trauma. 2006;61:1285–1291. doi: 10.1097/01.ta.0000240969.13891.9b. [DOI] [PubMed] [Google Scholar]
- 43.Perl M, Gebhard F, Braumuller S, Tauchmann B, Bruckner UB, Kinzl L, Knoferl MW. The pulmonary and hepatic immune microenvironment and its contribution to the early systemic inflammation following blunt chest trauma. Crit. Care Med. 2006;34:1152–1159. doi: 10.1097/01.CCM.0000207343.53990.A8. [DOI] [PubMed] [Google Scholar]
- 44.Abraham E. T- and B-cell function and their roles in resistance to infection. New Horiz. 1993;1:28–36. [PubMed] [Google Scholar]
- 45.Gomez F, Ruiz P, Bernal JA, Escobar M, Garcia-Egido A, Lopez-Saez JJ. Enhancement of splenic-macrophage Fcγ receptor expression by treatment with estrogens. Clin. Diagn. Lab. Immunol. 2001;8:806–810. doi: 10.1128/CDLI.8.4.806-810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 47.Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF-α- and Fas-mediated apoptosis through NK-κB activation. Am. J. Physiol. 2001;281:G1357–G1368. doi: 10.1152/ajpgi.2001.281.6.G1357. [DOI] [PubMed] [Google Scholar]