Abstract
Objective
The biologic explanation for fetal receptivity to donor engraftment and subsequent long-term tolerance following transplantation early in gestation is not known. We investigated the role fetal immune ontogeny might play in fetal transplantation tolerance in sheep.
Methods
Engraftmentof allogeneic and xenogeneicHSC was determined 60 days following transplantation at different time points in sheep fetal gestation. Parallel analysis of surface differentiation antigen expression on cells from lymphoid organs of timed gestational age fetal sheep was determined by flow cytometry using available reagents.
Results
An engraftment window was identified after day 52 gestation lasting until day 71 (term gestation: 145 days). This period was associated with the expression of the leukocyte common antigen CD45 on all cells in the thymus. Double-positive and single-positive CD4 and CD8 cells began appearing in the thymus just prior (day 45 gestation) to the beginning of the engraftment window, while single-positive CD4 or CD8 cells do not begin appearing in peripheral organs until late in the engraftment period, suggesting deletional mechanisms may be operative. In concert, surface IgM-positive cells express CD45 in the thymus at day 45, with a comparable delay in the appearance of IgM/CD45 cells in the periphery until late in the engraftment window.
Conclusions
These findings support a central role for the thymus in multilineage immune cell maturation during the period of fetal transplantation receptivity. Further, they suggest that fetal engraftment receptivity is due to gestational age-dependent deletional tolerance.
Key Words: Fetal transplantation tolerance, Fetal immune ontogeny, Deletional tolerance
Introduction
The therapeutic application of in utero hematopoietic stem cell transplantation(IUHSCT) is theoretically attractive for definitive treatment of congenital disease states. Investigating this technique in sheep, we have shown long-term engraftment and expression of both allogeneic and xenogeneic donor cells without cytoablation and, under appropriate conditions, without graft-versus-host disease [1]. This has proved useful in identifying and characterizing the hematopoietic stem cell (HSC) compartment [2, 3]. We have demonstrated the ability to expand the hematopoietic graft after birth [4] and, in a similar fashion, demonstrated the feasibility of gene transfer using this large animal model [5].
The theoretical basis for IUHSCT is the well-recognized immune receptivity of the fetus to engraftment of donor cells [6]. Engraftment receptivity and long-term donor expression are characteristics of transplantation in sheep when performed at the appropriate time in gestation [7, 8]. For example, following application of skin grafts to fetal sheep at various gestational ages, skin graft acceptance after birth is noted if skin grafting is performed prior to day 71 gestation (term gestation: 145 days) [9]. Further, following in utero gene transfer into sheep fetuses of d55–d60 gestation, specific non-responsiveness of peripheral blood (PB) mononuclear cells and lack of inducible specific antibody to the gene product can be demonstrated after birth [10]. Investigations using alternate animals have also noted that gestational age at transplantation is critical to the development of immune tolerance and, as a consequence, long-term engraftment [6, 11]. It is interesting to note that this timing of IUHSCT varies depending on the size of the host animal [1,12,13,14].
To gain insight into the relationship between fetal immune ontogeny and the development of long-term transplantation tolerance following IUHSCT, we performed parallel experiments detailing engraftment receptivity in fetal sheep and the appearance of immune phenotypes in sheep fetal organs at varying gestational ages (day 39 to birth). Engraftment receptivity was determined to both allo- and xenogeneic donor HSC, while immune phenotypes were determined by flow cytometry using available surface antibodies. Engraftment receptivity is absent prior to day 52 gestation, peaks between days 64 and 71 gestation and then rapidly declines. Early gestation is characterized by delayed expression of the leukocyte common antigen (LCA) CD45 on all lineages in all organs except the thymus. Double-positive and single-positive CD4/8 cells appear in the thymus at day 45 of gestation. Single-positive CD4 and CD8 surface-bearing cells are not seen in peripheral organs until late in the period of engraftment receptivity, suggesting deletional mechanisms predominate. In a similar fashion, surface IgM (sIgM)-positive cells in the thymus express CD45 early, followed by those in PB and spleen. These findings support a central role for the thymus in multilineage immune cell maturation during the period of fetal transplantation receptivity, and suggest long-term engraftment and expression following IUHSCT is due to gestational age-dependent deletional tolerance. Further, it suggests that IUHSCT in humans should be performed during the comparable period in human gestation.
Materials and Methods
Timed Gestational Age Determination for Transplantation and Organ Acquisition
Merino Rambouillet ewes were estrus-synchronized and exposed to a ram for a 3-day period. Conception date (±1.5 days) was confirmed by ultrasound. Flow cytometry experiments also assayed for pregnancy-specific protein B (Biotracking). Pregnant ewes were sacrificed; the fetus was removed and lung, thymus, spleen, bone marrow, intestine, and PB were collected in saline. Single cell suspensions were obtained by homogenizing the tissues in PBS + 10% FBS.
In utero HSC Transplantation
Allotransplantation
4 × 106 T-cell-depleted sheep cord blood mononuclear cells [15] were transplanted into 3 fetal recipients of the opposite sex at each specified gestational age as previously described [7], except day 71 recipients. Day 71 recipient sheep (n = 3) were homozygous type B hemoglobin. These were transplanted with 4 × 106 T-cell-depleted sheep cord blood mononuclear cells from hemoglobin type A donors.
Engraftment was determined following aspiration of fetal bone marrow 60 days after transplantation. Y chromosome determinations were used to arrive at donor cell engraftment values for the opposite sex transplantations while the presence of type A hemoglobin determined engraftment in the day 71 fetuses [7]. The Institutional Animal Care and Use Committee of the University of Nevada, Reno, approved all protocols.
Xenotransplantation
2 × 106 normal human bone marrow CD34+ cells were isolated as previously described [16] and transplanted into each fetal recipient at the specified gestational age as above. Four fetuses were transplanted at each gestational age. The University of Nevada, Reno, Biomedical Human Subjects Committee approved the human stem cell transplantation protocol.
Engraftment was determined on bone marrow cell suspensions following aspiration 60 days after transplantation by the presence of surface human CD45 as previously described [8].
For both allo- and xenotransplantation, cells were transplanted at gestational ages 35, 40, 47, 52, 58, 64, 71, 80, and 92.
Antibodies
Murine monoclonal antibodies to ovine cell surface antigens CD4, CD8, CD25, and CD45 (from University of Melbourne, Australia); the following preconjugated murine anti-ovine antibodies obtained from Serotec (Raleigh, N.C., USA) were used in flow cytometric analysis: CD1:FITC, CD1:Alexa647, CD4:PE, CD8:FITC, CD45:PE, CD45:FITC, and IgM:FITC. For secondary labeling, rat anti-mouse IgG1:PE and rat anti-mouse IgG2a:FITC obtained from BD Pharmingen (San Diego, Calif., USA) were used as secondary antibodies and isotype control. Conjugated murine IgG1/IgG2 FITC/PE also obtained from BD Pharmingen served as isotype control for conjugated CD1, CD4, CD8, CD45 and IgM.
Cell Staining and Flow Cytometry
Following red blood cell lysis, the resulting single cell suspensions (1 × 106 cells) were incubated with FcR blocking reagent (Miltenyi Biotec) per manufacturer's protocols, and then stained with primary antibody for 30 min, washed with PBS + 0.1% sodium azide, stained with secondary antibody for 15 min, washed with PBS + 0.1% sodium azide and fixed in Streck Cytometry Sheath fluid (Streck Laboratories) +1% formaldehyde. Conjugated (PE or FITC or Alexa647) antibodies were either added at the time of secondary labeling or with an additional 15 min incubation and wash with PBS + 0.1% sodium azide just prior to fixing. The data was collected on a Becton-Dickinson FACScan and analyzed using CellQuest software. Quadrants were individually determined for each organ/PB to establish isotype binding for both primary and secondary isotype controls at less than 5%. 10,000 events were counted using a wide acquisition gate while eliminating dead cells on the basis of forward light scatter, except in early gestational age fetuses with small gross sample sizes where a minimum of 1,000 events were analyzed. Data points with <1,000 events were not included in further analyses. Cumulative data is presented in terms of event number rather than percent expression. This more clearly reflects the linear and exponential growth phases of the organs presented.
For cumulative CD45 measurements, for day 39, n = 1 (n = 2 thymus), day 45, n = 4 (n = 3 spleen, n = 2 PB), day 52, n = 4 (n = 2 spleen), day 58, n = 4, day 65, n = 9 (n = 8 PB), day 80, n = 3 (n = 2 PB), day 85, n = 4. For cumulative CD4/8 measurements, for day 39, n = 2 (n = 1 PB), day 45, n = 4 (n = 2 PB), day 52, n = 3 (n = 1 spleen), day 58, n = 3, day 65, n = 6, day 80, n = 3. For cumulative IgM measurements, for day 39, n = 2, day 45, n = 4 (n = 3 thymus, n = 2 PB), day 52, n = 4 (n = 2 spleen), day 58, n = 4, day 65, n = 7 (n = 6 PB), day 80, n = 3, day 85, n = 4.
Results
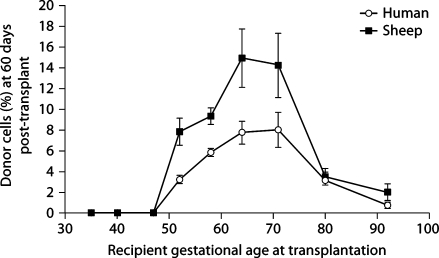
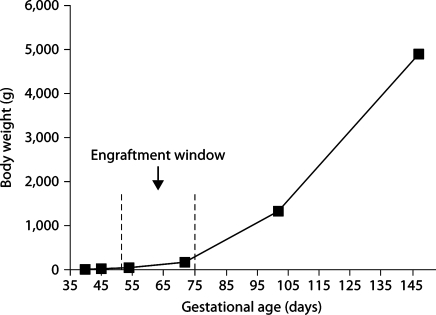
Figure 1 presents engraftment receptivity to allogeneic and xenogeneic donor HSC. Engraftment was determined by assaying the bone marrow 60 days after transplant and was not seen prior to day 52 gestation. Independent of donor source, donor cell expression peaks when transplantation is performed between days 64 and 71 of gestation and then rapidly falls. This period of engraftment receptivity is consistent with fetal skin graft receptivity as demonstrated by Silverstein et al. [9]. The engraftment window takes place during the late embryonic phase of gestation (first trimester) when growth is relatively linear, in comparison to the fetal stage (second and third trimesters) where growth is logarithmic (fig. 2). The body weight change during the engraftment window is 150 g, while after the window closes the fetus gains 4.7 kg. This is similar to the body weight change noted in humans [17].
Fig. 1.
Engraftment receptivity is gestational age-dependent. For both allo- and xenotransplantation, cells were transplanted at gestational ages 35, 40, 47, 52, 58, 64, 71, 80, and 92. Independent of donor source, there is an absence of engraftment if IUHSCT is performed prior to day 52, receptivity peaks between days 64 and 71 of gestation and then rapidly falls. SEM is shown as error bars.
Fig. 2.
Sheep body weight during gestation and at birth in relation to engraftment window. The engraftment window occurs during a period of relatively flat growth.
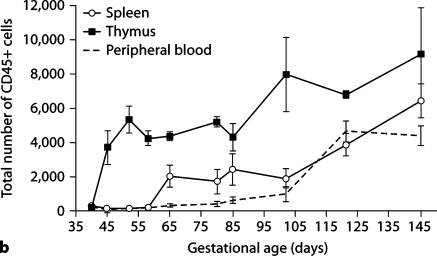
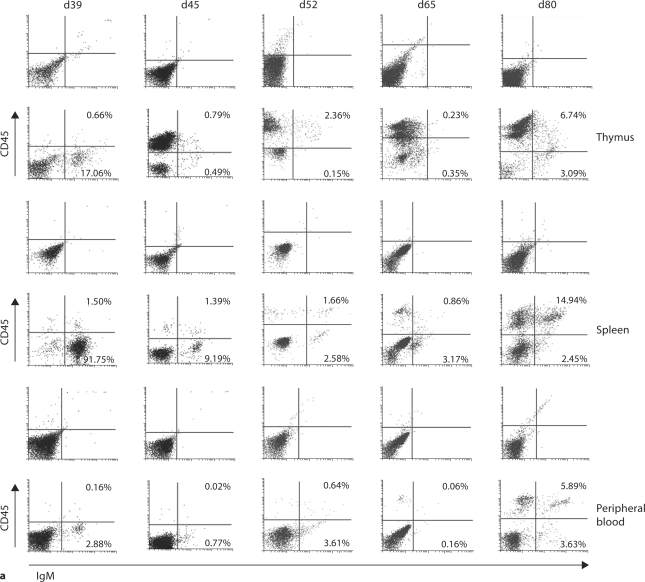
Figure 3a presents representative histogram readings of total CD45 expression on cells from sheep thymus, spleen, PB, bone marrow, lung, and small intestine at the designated gestational time points. CD45 expression is noted earliest in the thymus, with a delay of up to 20 days before expression in spleen and PB towards the end of the engraftment window. Appreciable expression of CD45 on cells from bone marrow, lung and small intestine occurs after the engraftment window closes, and therefore our further studies focused solely on thymus, spleen, and PB. In figure 3b, we present cumulative data on the number of cells expressing CD45 in the thymus, spleen and PB at various gestational ages. Again, early expression of CD45 occurs in the thymus. The period of engraftment receptivity outlined in figure 1 coincides with the appearance of CD45 (the leukocyte common antigen) on all cells in the thymus in comparison to its delayed expression on cells in other organs.
Fig. 3.
Expression of CD45 (LCA) on cells of thymus and peripheral organs during gestation. Expression is found early in thymus (beginning at day 52), followed by expression in spleen and PB after the engraftment window closes (day 80). a Representative histograms showing percent expression of CD45 on fetal ovine thymus, PB, spleen, bone marrow, lung, and small intestine at selected time points throughout gestation. b Cumulative data demonstrating expression of CD45 on cells from fetal ovine thymus, spleen, and PB throughout gestation. SEM is shown as error bars.
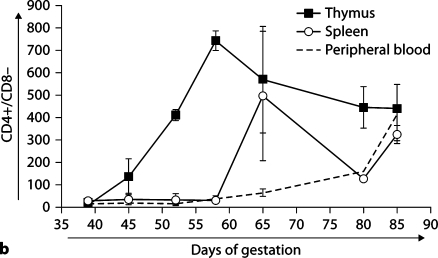
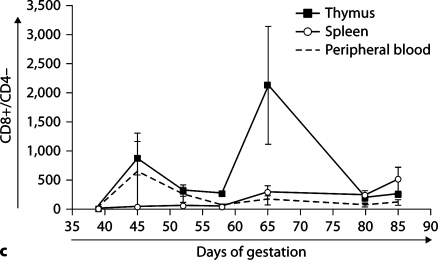
Figure 4 presents CD4 and CD8 surface expression data on cells from thymus, spleen, and PB. Figure 4a shows representative dot plots of expression of CD4 and CD8 in thymus, spleen, and PB throughout early to mid gestation, while expression profiles of single-positive CD4 (fig. 4b) and CD8 (fig. 4c) cells are shown as cumulative data. At day 39, no CD4- or CD8-expressing cells are seen, suggesting the absence of T-cell maturation prior to day 45. As gestation progresses, active maturation is noted in the thymus with the generation of thymic double-positive and single-positive CD4 and CD8 cells. However, few single-positive CD4 or CD8 cells are seen in the periphery. Expression of single-positive CD4 and CD8 cells is more delayed in the other organs tested (data not shown). This period coincides with the demarcation of the thymus into cortex and medulla at day 42 gestation [18].
Fig. 4.
CD4 and CD8 expression on ovine fetal organs during gestation. Thymic T-cell maturation is absent at day 39, but begins with the appearance of double-positive CD4+/CD8+ and singlepositive CD4 and CD8 cells by day 45, while the appearance of single-positive CD4 and CD8 cells is delayed in the periphery until the end of the engraftment window. Dot plots of representative samples are presented in a. Quadrants were individually determined based on isotype control binding for each organ at each gestational age. Primary isotype controls are shown. Cumulative data showing the presence of CD4+/CD8– (b), and CD8+/CD4– (c) in thymus, spleen, and PB during early and mid gestation. SEM is shown as error bars.
Figure 5 presents maturation profiles of sIgM-positive cells in thymus, spleen, and PB. CD45 expression is delayed on splenic and PB sIgM-positive cells in comparison to thymic sIgM-positive cells. As with the CD4/8 cells, the presence of sIgM-positive cells expressing CD45 is further delayed in the other organs examined.
Fig. 5.
CD45 expression on surface IgM+ cells in ovine fetal thymus, spleen, and PB during gestation. Surface IgM expression is noted earliest in thymus followed by spleen and PB. a Dot plots showing expression of CD45 and sIgM in thymus, spleen, and PB. Quadrants were individually determined based on isotype control binding for each organ at each gestational age. Primary isotype controls are shown. b Cumulative data on CD45 expression of sIgM+ on thymic and splenic cells.
With regard to figures 4c and 5b, the difference between thymic and splenic CD8 and IgM/CD45 cells is small in relative numbers, yet these small differences in cell numbers support a sieving ratio of 80%. Moreover, this sieving ratio likely expands in concert with the exponential (fetal) growth phase of the thymus (fig. 6).
Fig. 6.
Sheep thymic weight during gestation in relation to engraftment window.
Discussion
Immunity is characterized by the ability to recognize and then apply effector mechanisms to neutralize invading microorganisms. In general, this process involves interaction of antigen presenting cells and antigen with T and B cells, resulting in an effector response. Tolerance involves inhibition to limit this effector response, with formation of the tolerance repertoire occurring during fetal development [19]. While our knowledge is incomplete, the processes likely involve antigen presentation, deletion of reactive clones, anergy and/or cellular suppression [20, 21].
The CD45 antibody we used recognizes isoforms of MW 225, 210, and 190 kDa. We believe the appearance of CD45 represents differentiation of lineage-specific immune cells from the immature CD45RABC isoform (MW: 240 kDa), which is not detectable by presently available reagents. While unable to rule out the unlikely possibility that cells not expressing CD45 are expressing the fully differentiated isoform CD45RO, estimated to weigh 180 kDa [22, 23], we believe fetal immune ontogeny is characterized by the differentiation of all lineage-specific cells (CD1 or APC data not shown) in the thymus with subsequent distribution elsewhere, perhaps by migration from the thymus and/or expansion. Early in gestation, this is most noticeable in the spleen.
These descriptive studies in a large animal, although preliminary, permit temporal understanding of organ-specific events in early immune ontogeny and their likely association with the observed engraftment window. We believethe most probable explanation for the delay in the appearance of single-positive CD4 and CD8 cells in the periphery is deletion of single-positive cells in the thymic medulla. As gestation progresses, single-positive CD4 and CD8 cell are capable of escaping the deletional sieve and begin appearing in the periphery. In a comparable fashion, in figure 5 we note early differentiation (implied by expression of CD45) of sIgM cells in the thymus, and their subsequent appearance in the spleen and PB.
The engraftment window is transient with both a beginning, where transplantation prior to its onset will not result in engraftment, nor, after its passing, will engraftment occur. We examined engraftment of T-cell-depleted allogeneic marrow and engraftment of CD34-enriched human marrow cells. It is not possible to compare the differences in levels of engraftment in view of the disparity in the different preparations. It is possible that lack of engraftment niche(s) in the marrow account for the failure of HSC to engraft prior to day 52 [24]. However, we think it more likely that the establishment of the thymic medulla (and its deletional function) accounts for the beginning of the engraftment receptivity window [18, 25, 26]. Further, these observations support a model of deletional imprinting and memory that are gestational age-dependent and involve CD45 modulation [27]. As B-cell tolerance is noted following gene transplantation during the engraftment window and early differentiation (expression of CD45) of B cells is evident in the thymus, we believe the most likely mechanism to account for B-cell tolerance following fetal transplantation is thymic imprinting of deletional memory into the B lineage as well as the T lineage. While we believe the mechanism of B-cell tolerance is deletion, as can readily be seen in the CD4 lineage (fig. 4b), it is possible that B-cell tolerance is mediated via its known APC function [28, 29]. This large animal model offers advantages in long-term monitoring of donor engraftment and allows this glimpse into fetal immune ontogeny that would not be possible in a small animal. Studies using genetically defined mice support the assertion that engraftment following fetal transplantation is due in part to deletional tolerance to donor cells [14].
In addition, the period of gestational transplant receptivity in sheep takes place during the late embryonic/early fetal stage of gestation prior to or at the onset of exponential fetal growth as shown in figure 2; yet fetal transplantation tolerance is not lost. Therefore, the mechanisms that underlie thymic-derived tolerance formed during the engraftment window, characterized by the early appearance of CD45, must be expandable, renewable and exportable to the rest of the body prior to birth [30,31,32,33].
Fetal transplantation studies in mice demonstrate conflicting data with regard to tolerance mechanisms [14,34,35,36]. This may be due to the proposed brief period for establishment of deletional tolerance in mice (likely <48 h) [37]. In large mammals with long gestation periods (145 days sheep/270 days humans), the engraftment window may approach 20–30 days and occurs prior to exponential growth. Our data would support performing fetal transplantation in humans early in the second trimester. This would be after week 12, when the demarcation of the thymic cortex and medulla occur, but prior to the appearance of T cells in the spleen at week 16, since thymic demarcation occurs somewhat earlier in human (31.5 vs. 35% expiration of gestation) than in sheep gestation [38, 39].
In summary, engraftment receptivity and immune ontogeny were studied in fetal sheep. We identified an engraftment window (days 51–71 gestation) to allogeneic and xenogeneic donor HSC. The window is associated temporally with the establishment of the thymic medulla and the onset of multilineage CD45 isoform differentiation in the thymus. Central deletion is supported as the underlying mechanism for fetal long-term engraftment receptivity by the absence of maturing cells in the periphery, as evidenced by the lack of CD45 expression. These observations support a model for deletional tolerance that requires fetal imprinting of long-term deletional memory. Implications with regard to performing in utero stem cell and gene transplantation in humans to treat congenital diseases are discussed.
Acknowledgements
Grant acknowledgements: NIH: HL52955, HL66058, HL49042, DK67786.
References
- 1.Pixley JS, Mackintosh FR, Zanjani ED. Experimental and clinical basis of intrauterine stem cell transplantation. Rev Clin Exp Hematol. 1999;8:11–32. [Google Scholar]
- 2.Zanjani ED, Almeida-Porada G, Livingston AG, Zeng H, Ogawa M. Reversible expression of CD34 by adult human bone marrow long-term engrafting hematopoietic stem cells. Exp Hematol. 2003;31:406–412. doi: 10.1016/s0301-472x(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 3.Zanjani ED. The human sheep xenograft model for the study of the in vivo potential of human HSC and in utero gene transfer. Stem Cells. 2000;18:151. doi: 10.1634/stemcells.18-2-151. [DOI] [PubMed] [Google Scholar]
- 4.Almeida-Porada G, Porada C, Gupta N, Torabi A, Thain D, Zanjani ED. The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts' potential. Exp Hematol. 2007;35:1594–1600. doi: 10.1016/j.exphem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porada CD, Tran N, Eglitis M, Moen RC, Troutman L, Flake AW, Zhao Y, Anderson WF, Zanjani ED. In utero gene therapy: transfer and long-term expression of the bacterial neo(r) gene in sheep after direct injection of retroviral vectors into preimmune fetuses. Hum Gene Ther. 1998;9:1571–1585. doi: 10.1089/hum.1998.9.11-1571. [DOI] [PubMed] [Google Scholar]
- 6.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 7.Flake AW, Harrison MR, Adzick NS, Zanjani ED. Transplantation of fetal hematopoietic stem cells in utero: the creation of hematopoietic chimeras. Science. 1986;233:776–778. doi: 10.1126/science.2874611. [DOI] [PubMed] [Google Scholar]
- 8.Zanjani ED, Pallavicini MG, Ascensao JL, Flake AW, Langlois RG, Reitsma M, Mackintosh FR, Stutes D, Harrison MR, Tavassoli M. Engraftment and long-term expression of human fetal hemopoietic stem cells in sheep following transplantation in utero. J Clin Invest. 1992;89:1178–1188. doi: 10.1172/JCI115701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverstein A, Prendergast RA, Kraner K. Fetal response to antigenic stimulus. IV. Rejection of skin homografts by the fetal lamb. J Exp Med. 1964;119:955–964. doi: 10.1084/jem.119.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran ND, Porada CD, Almeida-Porada G, Glimp HA, Anderson WF, Zanjani ED. Induction of stable prenatal tolerance to β- galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood. 2001;97:3417–3423. doi: 10.1182/blood.v97.11.3417. [DOI] [PubMed] [Google Scholar]
- 11.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin NM. Tolerance induced by thymic epithelial grafts in birds. Science. 1987;237:1032–1035. doi: 10.1126/science.3616623. [DOI] [PubMed] [Google Scholar]
- 12.Lutzko C, Meertens L, Li L, Zhao Y, Abrams-Ogg A, Woods JP, Kruth S, Hough MR, Dube ID. Human hematopoietic progenitors engraft in fetal canine recipients and expand with neonatal injection of fibroblasts expressing human hematopoietic cytokines. Exp Hematol. 2002;30:801–808. doi: 10.1016/s0301-472x(02)00830-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee PW, Cina RA, Randolph MA, Goodrich J, Rowland H, Arellano R, Kim HB, Sachs DH, Huang CA. Stable multilineage chimerism across full MHC barriers without graft-versus-host disease following in utero bone marrow transplantation in pigs. Exp Hematol. 2005;33:371–379. doi: 10.1016/j.exphem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Peranteau WH, Hayashi S, Hsieh M, Shaaban AF, Flake AW. High-level allogeneic chimerism achieved by prenatal tolerance induction and postnatal non-myeloablative bone marrow transplantation. Blood. 2002;100:2225–2234. doi: 10.1182/blood-2002-01-0166. [DOI] [PubMed] [Google Scholar]
- 15.Crombleholme TM, Harrison MR, Zanjani ED. In utero transplantation of hematopoietic stem cells in sheep: the role of T cells in engraftment and graft-versus-host disease. J Pediatr Surg. 1990;25:885–892. doi: 10.1016/0022-3468(90)90197-h. [DOI] [PubMed] [Google Scholar]
- 16.Pixley JS, Zanjani ED, Shaft DM, Porada C, Mackintosh FR. Prolonged hematopoietic chimerism in normal mice transplanted in utero with human hematopoietic stem cells. Pathobiology. 1998;66:230–239. doi: 10.1159/000028028. [DOI] [PubMed] [Google Scholar]
- 17.Carlson BM. Human Embryology and Development. New York: Churchill Livingstone; 1999. Fetal period and birth; pp. 447–468. [Google Scholar]
- 18.Maddox JF, Mackay CR, Brandon MR. Ontogeny of ovine lymphocytes. I. An immunohistological study on the development of T lymphocytes in the sheep embryo and fetal thymus. Immunology. 1987;62:97–105. [PMC free article] [PubMed] [Google Scholar]
- 19.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 20.Cancro MP. Crossing the threshold of lymphocyte activation. J Immunol. 2005;174:5159–5160. doi: 10.4049/jimmunol.174.9.5159. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz RH, Mueller DL. Immunological tolerance. In: Paul WE, editor. Fundamental Immunology. New York: Lippincott-Raven Press; 1999. pp. 901–963. [Google Scholar]
- 22.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 23.Maddox JF, Mackay CR, Brandon MR. The sheep analogue of leucocyte common antigen. Immunology. 1985;55:347–353. [PMC free article] [PubMed] [Google Scholar]
- 24.Flake AW, Zanjani ED. In utero hematopoietic stem cell transplantation: ontogenic opportunities and biologic barriers. Blood. 1999;94:2179–2191. [PubMed] [Google Scholar]
- 25.Laufer TM, Glimcher LH, Lo D. Using thymus anatomy to dissect T-cell repertoire selection. Semin Immunol. 1999;11:65–70. doi: 10.1006/smim.1998.9997. [DOI] [PubMed] [Google Scholar]
- 26.Naquet P, Naspetti M, Boyd R. Development, organization and function of the thymic medulla in normal, immunodeficient or autoimmune mice. Semin Immunol. 1999;11:47–55. doi: 10.1006/smim.1998.0149. [DOI] [PubMed] [Google Scholar]
- 27.Trop S, Charron J, Arguin C, Lesage S, Hugo P. Thymic selection generates T cells expressing self-reactive TCRs in the absence of CD45. J Immunol. 2000;165:3073–3079. doi: 10.4049/jimmunol.165.6.3073. [DOI] [PubMed] [Google Scholar]
- 28.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Roux E, Dumont-Girard F, Starobinski M, Siegrist CA, Helg C, Chapuis B, Roosnek E. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–2303. [PubMed] [Google Scholar]
- 31.De Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–1702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 32.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 33.Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nat Clin Pract Rheumatol. 2006;2:44–52. doi: 10.1038/ncprheum0049. [DOI] [PubMed] [Google Scholar]
- 34.Carrier E, Lee TH, Busch MP, Cowan MJ. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood. 1995;86:4681–4690. [PubMed] [Google Scholar]
- 35.Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor-specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surg. 1999;34:726–729. doi: 10.1016/s0022-3468(99)90364-0. [DOI] [PubMed] [Google Scholar]
- 36.Sefrioui H, Donahue J, Srivastava AS, Gilpin E, Lee TH, Carrier E. Alloreactivity following in utero transplantation of cytokine-stimulated hematopoietic stem cells: the role of recipient CD4– cells. Exp Hematol. 2002;30:617–624. doi: 10.1016/s0301-472x(02)00803-2. [DOI] [PubMed] [Google Scholar]
- 37.Fairchild PJ, Waldmann H. Extrathymic signals regulate the onset of T-cell repertoire selection. Eur J Immunol. 2000;30:1948–1956. doi: 10.1002/1521-4141(200007)30:7<1948::AID-IMMU1948>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Larsen WJ. Human Embryology. Cincinnati: Churchill Livingstone; 1997. Development of the head and neck; pp. 347–374. [Google Scholar]
- 39.Stites DP, Pavia CS. Ontogeny of human T cells. Pediatrics. 1979;64:795–802. [PubMed] [Google Scholar]