Abstract
Many herbs have been used as therapeutics in Korean traditional medicine. In view of their clinical indications, anti-oxidant activity may contribute to their pharmacological effects. However, anti-oxidant information on these plants has not been available. In this study, seventy herbs which have been used in Korean traditional medicine were selected and screened for anti-oxidant activity using their water extracts. The anti-oxidant activity was assessed by their ability to inhibit three oxidation reactions; luminol/Fenton reagent, 2, 7-dichlorodihydrofluorescein (DCHF)/Fenton reagent and DCHF/peroxynitrite. In each assay, 70 herbs were divided into two groups; anti-oxidant group which inhibited the respective oxidation reaction and was majority (about 60 herbs), and pro-oxidant group which enhanced the oxidation reaction but was minority (more or less 10 herbs). When the herbs were listed in the order of their anti-oxidant strength, the orders obtained from each assay were found to be quite similar. The upper top rankers (more or less 10 herbs) in each assay showed strong activity compared to the others. The uppermost rankers in each assay were Rubus coreanus Miquel/ Rubus schizostylus, Schisandra chinensis Baillon/ Schizandra chinensis and Terminalia chebula Retzius/ Terminalia chebula. Of the pro-oxidant herbs, about 4-5 herbs were strongly pro-oxidant, which enhanced the control oxidation reactions to 150-300%. But the meaning of this observation is not known since few of them in one assay were also anti-oxidant in other assays. The results obtained in the present study may serve as information for understanding pharmacological effects of these herbs and developing new drugs from them.
Keywords: Anti-oxidants, herbs, chemiluminescence, peroxynitrite, Fenton reagent
Introduction
Free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced as byproducts in aerobic metabolism, and have been implicated in the pathogenesis of many diseases, which include cancer, atherosclerosis, diabetes mellitus, hypertension, inflammation and aging (Bagchi et al., 1995; Halliwell & Gutteridge, 1984; 1Lee et al., 2000a; Wallace, 1999).
Nature has provided man with antioxidant defense system, which is an armamentarium with enzymes and compounds that can remove free radicals (Catapano et al., 2000; Eder et al., 2002; Libby, 2002). Imbalance between production and elimination of free radicals leads to oxidative stress, which damages cells and eventually causes diseases. Therefore, maintenance of antioxidant activity is important in prevention of the above mentioned free radical-associated diseases and aging.
Many plants have been used for centuries in Korean traditional medicine as anti-inflammatory agents, analgesics, emmenagogues, antispasmodics, sedatives or health-improving agents (Bent & Ko 2004; Liu, 2003; Zanon et al., 1999). These therapeutic uses suggest that the diseases for which these herbal plants were used appear to be associated with oxidative stress and thus, anti-oxidant action may play some roles in their therapeutic actions. A large number of substances of plant origin have been found to act as antioxidants by scavenging ROS and RNS, and some of them have therapeutic potentials for free radical associated disorders (Hausladen & Stamler, 1999; 2Lee et al., 2000b). Therefore, it is meaningful to assess anti-oxidant activity of the plants used in the herbal medicine either to elucidate the mechanism of their pharmacological actions or to provide information on anti-oxidant activity of these herbal plants.
In the present study, 70 herbs that have been used traditionally in Korean herbal medicine were selected and evaluated for their antioxidant activities. The anti-oxidant activity was assessed using water extracts of these plants because when these plants are used for patients, infusions prepared by boiling them in water are given to patients.
Materials and Methods
Chemicals
Ferrous chloride hexahydrate and hydrogen peroxide (H2O2) were purchased from Kanto Chemical, and 5-amino-2, 3-dihydro-1, 4-phthalazinedione (luminol) and 2, 7-dichlorodihydrofluorescein (DCHF) were from Sigma and sodium peroxynitrite from Cayman.
Herbs
Seventy herbs were selected from the literatures describing pharmacological actions and clinical uses of plants (Nakatani, 2000; Zheng & Wang, 2001; Zhu, 1998) and obtained from Kyung Dong herbal market in Seoul. The herbal plants purchased were identified by Dr. Gyu-Mahn Jeong at the Botanical Garden, Kyunghee University. Herbarium voucher specimens were prepared and deposited at the herbarium of the Professional Graduate School of Oriental Medicine, Kyunghee University in Seoul.
Water extracts of herbs
Parts of each herb used for the patients in the traditional medicine such as leaves, roots, flowers, seeds, fruits, barks or sclerotium of each herbal plant were dried and crushed. One hundred grams of the crushed part was placed into 1 liter of distilled water and boiled for 3 hours. Water was then collected by filtration. The remaining herb residue was boiled again in 1 liter of newly added water for 3 hours and then water was collected by filtration. The two water parts collected by filtration were combined, concentrated to 10 ml and filtered through a 0.45 µm Millipore filter. The filtrate was used as a water extract for assessing the anti-oxidant activity of the herb.
Anti-oxidant activity assay using chemiluminescence
Anti-oxidant activity of each water extract was assayed by its ability to inhibit chemiluminescence produced from luminol on its oxidation by H2O2/Fe++ (Fenton reaction) (Zhu et al., 1994). Briefly, luminol (10 mM) was mixed with 30 mM H2O2, 0.5 mM FeCl2 and PBS, pH 7.4 in the absence or presence of various volumes of each water extract. Total volume was 2 ml. Reaction was started by adding H2O2 last and allowed at 37℃. After 10 min, chemiluminescence was measured using a chemiluminescence analyzer (Biolumet LB 9505, Berthold, Germany). In a preliminary experiment, control chemiluminescence (produced in the absence of the herbal extracts) was linearly increased up to 10 min and thus the chemiluminescence measured at 10 min was used for the comparison of anti-oxidant activities. The anti-oxidant activity was expressed by a reciprocal of the volume of the water extract required to inhibit the control chemiluminescence to 50% (1/50% inhibitory volume; 1/IV50).
Anti-oxidant activity assay using oxidation of DCHF by Fenton reagent
Anti-oxidant activity may differ depending upon assay systems used and thus, to get correct results, it should be assayed by more than one assay system. Therefore, each water extract was also assessed by fluorescence produced from DCHF (2, 7-dichloro-dihydrofluorescein) on its oxidation by Fenton reaction (Jakubowski & Bartoz, 2000). Briefly, 50 µM DCHF was mixed with 60 mM H2O2, 0.75 mM FeCl2 and PBS, pH 7.4 in the absence or presence of each water extract (5 µl) in 96 well plates. Total volume was 200 µl. Reaction was started by adding 60 mM H2O2 last, allowed at 37℃ for 10 min and then fluorescence was measured using a spectrofluorimeter (F-MAX-0200-1300, Molecular Devices) at ex. 485 nm and em. 535 nm. In a preliminary experiment, the control fluorescence (produced in the absence of water extract) was linearly increased up to 10 min and thus, the fluorescence was measured at 10 min after the reaction was started. The anti-oxidant activity was expressed by % inhibition of the control fluorecence [(control fluorescence-experimental fluorescence)/control fluorescence×100].
Anti-oxidant activity assay using oxidation of DCHF by peroxynitrite
Anti-oxidant activity of each water extract was assayed by another system, i.e. oxidation reaction of DCHF by sodium peroxynitrite. DCHF (0.5 mM), sodium peroxynitrite (0.5 mM) and sodium phosphate buffer (0.3 M) were incubated in the absence or presence of each water extract (5 µl) in 96-well plates at 37℃ for 10 min. Total volume was 200 µl. Reaction was started by adding sodium peroxynitrite and then fluorescence was measured using a spectrofluorometer (F-MAX-0200-1300, Molecular Devices) at ex. 485 nm and em. 535 nm. In a preliminary experiment, the control fluorescence (produced in the absence of water extract) was linearly increased up to 10 min and thus, the fluorescence was measured at 10 min after the reaction was started. The anti-oxidant activity was expressed by % inhibition of the control fluorescence [(control fluorescence-experimental fluorescence)/control fluorescence×100].
Statistical analysis
As described above, antioxidant activities of 70 herbs were measured by three assay systems; luminol/Fenton reagent, DCHF/Fenton reagent and DCHF/peroxynitrite. The reproducibility of antioxidant activity by each of the three assay systems were tested by intraclass correlation coefficients (ICC) using SPSS 12.0 computer program. In this analysis, the data of proxidant 11 herbs measured by luminol/Fenton reagent were excluded because the measured chemiluminescence values (108~109 range) were too large compared to those observed in other assay systems.
Results
Description of the herbal plants used in this study
Table 1 contains the information of the herbs used in the present study; names, voucher specimen number and parts of the plants used in the anti-oxidant assays. In Korean traditional medicine, when these herbs are used for patients, parts shown in the Table 1 of the respective plants are boiled in water and infusions prepared are given to the patients orally. For the convenience, serial number was given to each herb.
Table 1.
Information on the herbal plants used in this study
Anti-oxidant activities assessed by Fenton reagent-induced chemiluminescence
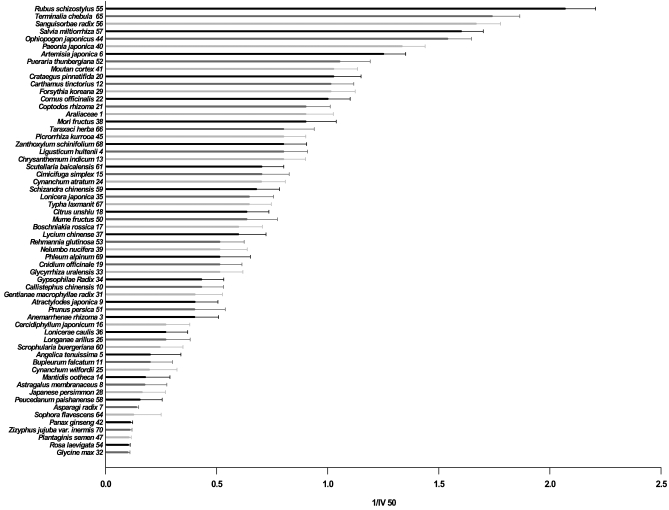
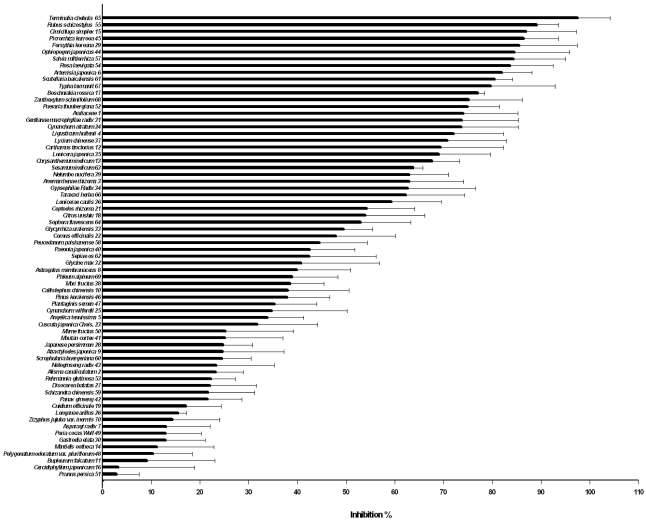
Firstly, anti-oxidant activities of the herbs were assessed by measuring their abilities to inhibit chemiluminescence emitting from luminol on its oxidation by Fenton reagent (H2O2/Fe++). Volume of water extract of each herb to inhibit chemiluminescence to 50% (IV50) was determined and the reciprocals of IV50 (1/IV50) of the respective herbs are shown in Fig. 1; the larger the value of 1/IV50 indicates the stronger its anti-oxidant activity. Of total 70 herbs, 60 suppressed the chemiluminescence and were grouped as anti-oxidant herbs although there were big differences in their activities. Interestingly, 10 herbs enhanced the chemiluminescence (Fig. 2) and were grouped as pro-oxidant since they accelerated the ROS-induced chemiluminescent reaction although the accelerating mechanism was not clearly explained. Of the anti-oxidant 60 herbs, 7 herbs in particular showed much higher anti-oxidant activities than the others, which were Rubus coreanus Miquel/ Rubus schizostylus〈55〉, Terminalia chebula Retzius/ Terminalia chebula〈65〉, Salvia miltiorrhiza Bunge/ Salvia miltiorrhiza〈56〉, Salvia miltiorrhiza〈57〉, Perilla frutescens L. Britton var. acuta (Thunb.) Kudo/ Ophiopogon japonicas〈44〉, Paeonia lactiflora Pallas/ Paeonia japonica 〈40〉, Artemisia annua Linne/ Artemisia japonica〈6〉, [the numbers in 〈 〉 are the serial numbers in Table 1]. On the other hand, of 10 herbs in Fig. 2, seven herbs exhibited the strongly enhanced chemiluminescence, which were Sesamum indicum Linne/ Sesamum indicum〈63〉, Cuscuta chinensis Lamark/ Cuscuta japonica Chois.〈23〉, Alisma orientale Juzepczuk/ Alisma canaliculatum〈2〉, Gastrodia elata Blume/ Gastrodia elata〈30〉, Polygonatum sibiricum Redoute/ Polygonatum odoratum var. pluriflorum〈48〉, Dioscorea batatas Decaisne/ Disocorea batatas〈27〉 and Sepia (Platysepia) esculenta Hoyle/ Sepiae os〈62〉.
Fig. 1.
Anti-oxidant activities of herbs used in Korean traditional medicine assessed by inhibition of chemiluminescence emitting from luminol/Fenton reagent reaction. Water extracts of seventy herbs were prepared and each of water extracts was assayed by measuring the inhibition of chemiluminscence produced from luminol on its oxidation by Fenton reaction. Luminol (10 mM) was mixed with 30 mM H2O2, 0.5 mM FeCl2 and phosphate, pH 7.4 in the absence or presence of various volumes of each water extract. Total volume was 2 ml and reaction was started by adding H2O2 last and allowed at 37℃. After 10 min, chemiluminescence was measured using a chemiluminescence analyzer. Of the 70 herbs, 60 were shown to inhibit the chemiluminescence. The anti-oxidant activity was expressed by a reciprocal of the volume of the water extract required to inhibit the control chemiluminescence observed in the absence of water extract to 50% (1/50% inhibitory volume; 1/IV50). 1/IV50 of 60 herbs are presented in this figure in the order of their magnitude. Numbers given at each herb are the serial numbers shown in Table 1.
Fig. 2.
Pro- oxidant activities of herbs used in Korean traditional medicine assessed by stimulation of chemiluminescence emitting from luminol/Fenton reagent reaction. The experimental conditions were the same as in Fig. 1. Of 70 herbs, 10 shown in the figure stimulated the chemiluminescence. The results are CPM (count per minute) of chemiluminescence by water extracts of each herb. Numbers given at each herb are the serial numbers shown in Table 1.
Anti-oxidant activities assessed by Fenton reagent-induced fluorescence
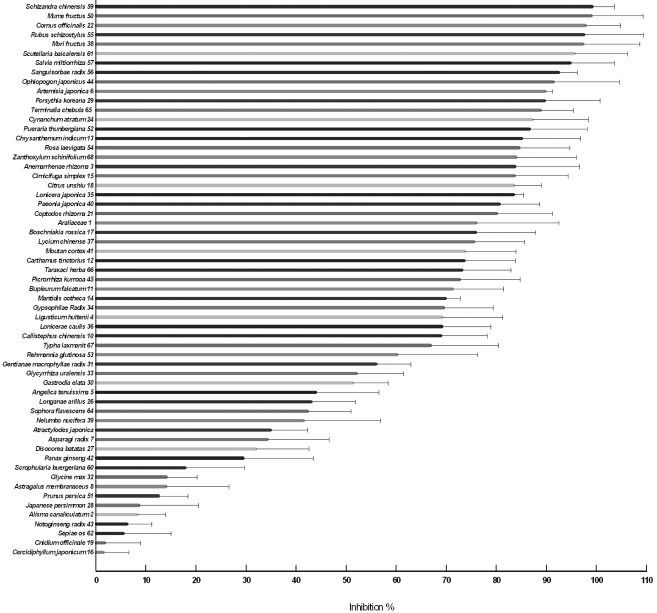
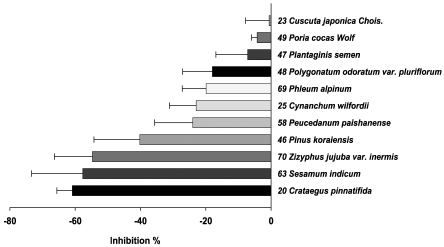
Secondly, anti-oxidant activities of the herbs were assessed by measuring their abilities to inhibit fluorescence produced from DCHF on its oxidation by Fenton reagent. The inhibition % of the fluorescence by the herbal extracts was determined and presented in the order of magnitude in Fig. 3. The higher inhibition % indicates the stronger anti-oxidant activity. Similar to the results of the chemiluminescence (Fig. 1 and 2), most of them (59 herbs) inhibited the fluorescence (Fig. 3) but some (11 herbs) enhanced the fluorescence (Fig. 4). The former were grouped as anti-oxidant and the latter as pro-oxidant although there were wide ranges in the inhibiting and enhancing activities, respectively. Of 59 herbs in Fig. 3, upper 10 showed more than 85% inhibition, which were Schisandra chinensis Baillon/ Schizandra chinensis〈59〉, Prunus mume Siebold et Zuccarini/ Mume fructus〈50〉, Cornus officinalis Siebold et Zuccarini/ Cornus officinalis〈22〉, Rubus coreanus Miquel/ Rubus schizostylus〈55〉, Morus alba Linne/ Mori fructus〈38〉, Scutellaria baicalensis Georgi/ Scutellaria baicalensis〈61〉, Salvia miltiorrhiza Bunge/ Salvia miltiorrhiza〈57〉, Sangusorba officinalis L./ Sanguisorbae radix〈56〉, Perilla frutescens L. Britton var. acuta (Thunb.) Kudo/ Ophiopogon japonicus〈44〉, Artemisia annua Linne/ Artemisia japonica〈6〉. Of 11 herbs in Fig. 4, five herbs showed significant enhancement of fluorescence, which were Crataegus pinnatifida Bunge var. typica Schneider/ Crataegus pinnatifida〈20〉, Sesamum indicum Linne/ Sesamum indicum〈63〉, Zizyphus jujuba Miller/ Phleum alpinum〈69〉, Pinus koraiensis Siebold et Zuccarini/ Pinus koraiensis〈46〉, Saposhnikovia divaricata Schiskin/ Peucedanum paishanense〈58〉.
Fig. 3.
Anti-oxidant activities of herbs used in Korean traditional medicine assessed by inhibition of fluorescence emitting from DCHF/Fenton reagent reaction. The anti-oxidant activities of 70 herbs were assessed using oxidation of DCHF by Fenton reagent. DCHF (50 µM) was mixed with 60 mM H2O2, 0.75 mM FeCl2 and PBS, pH 7.4 in the absence or presence of each water extract (5 µl) in 96 well plates. Total volume was 200 µl and reaction was started by adding 60 mM H2O2 last, allowed at 37℃ for 10 min and then fluorescence was measured. Of the 70 herbs, 59 were shown to inhibit the fluorescence. The anti-oxidant activity was expressed by % inhibition of the control fluorescence [(control fluorescence-experimental fluorescence)/control fluorescence×100]. Numbers given at each herb are the serial numbers shown in Table 1.
Fig. 4.
Herbs which stimulated fluorescence emitting from DCHF/Fenton reagent reaction. The experimental conditions were the same as in Fig. 3. Of the 70 herbs, 11 were shown to stimulate the fluorescence. The results are % stimulation by each herb. Numbers given at each herb are the serial numbers shown in Table 1.
Anti-oxidant activities assessed by peroxynitrite-induced fluorescence
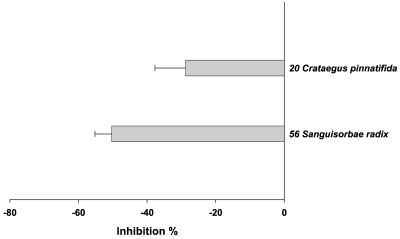
In addition to ROS, nitric oxide (NO.), a reactive nitrogen species (NOS), plays important roles in both physiological and pathological conditions. In pathological conditions NO. can damage cells in the form of ONOO- (peroxynitrite). Therefore, anti-oxidant activities of the herbs were assessed by their abilities to inhibit ONOO-induced oxidation. In this experiment, DCHF/ONOO- reaction was used. In Fig. 5, the inhibition % by each herb was presented in the order of the magnitude. In this assay system, all of the herbs except two inhibited the fluorescence and 8 herbs in particular showed more than 80% inhibition, which are Terminalia chebula Retzius/ Terminalia chebula〈65〉, Rubus coreanus Miquel/ Rubus schizostylus〈55〉, Cimicifuga heracleifolia Komarov/ Cimicifuga simplex〈15〉, Picrorrhiza kurroa Bentham/ Picrorrhiza kurrooa〈45〉, Forsythia viridissima Lindley/ Forsythia koreana〈29〉, Perilla frutescens L. Britton var. acuta (Thunb.) Kudo/ Ophiopogon japonicus〈44〉, Salvia miltiorrhiza Bunge/ Salvia miltiorrhiza〈57〉, Rosa laevigata Michaux/ Rosa laevigata〈54〉. The two herbs that enhanced the fluorescence were Sangusorba officinalis L./ Sanguisorbae radix〈56〉, Crataegus pinnatifida Bunge var. typica Schneider/ Crataegus pinnatifida〈20〉 (Fig. 6).
Fig. 5.
Anti-oxidant activities of herbs used in Korean traditional medicine assessed by inhibition of fluorescence emitting from DCHF/peroxynitrite. The anti-oxidant activities of the 70 herbs were assessed using oxidation of DCHF by peroxynitrite. DCHF (0.5 mM), sodium peroxynitrite (0.5 mM) and , sodium phosphate buffer (0.3 M) were incubated in the absence or presence of aech water extract (5 µl) in 96-well plates at 37℃ for 10 min. Total volume was 200 µl and reaction was started by adding sodium peroxynitrite and then fluorescence was measured using a spectrofluorimeter. Of the 70 herbs, 59 were shown to inhibit the fluorescence. The anti-oxidant activity was expressed by % inhibition of the control fluorescence [(control fluorescence-experimental fluorescence)/control fluorescence×100]. Numbers given at each herb are the serial numbers shown in Table 1.
Fig. 6.
Herbs which stimulated fluorescence emitting from DCHF/peroxynitrite reaction. The experimental conditions were the same as in Fig. 5. Of the 70 herbs, 2 were shown to stimulate the fluorescence. The results are % stimulation by each herb. Numbers given at each herb are the serial numbers shown in Table 1.
Discussion
In order to obtain more correct information, the anti-oxidant activity of 70 herbs was assessed by 3 oxidation reactions, which were luminal oxidation by Fenton reagent, DCHF oxidation by Fenton reagent and DCHF oxidation by peroxynitrite. Of the 70 herbs, most of them inhibited the oxidation reaction. It means that majority showed anti-oxidant activity. The anti-oxidant herbs selected by each assay were presented in Fig. 1, 2 and 5 in terms of the order of anti-oxidant strength. The results shown in each of three figures were not the same but showed significantly similar tendency (ICC for the data obtained from three assay systems; 0.506 (95% CI: 0.242~0.689). For example, the upper 10-15 rankers of high activity in one assay (ex. Fig. 1) were also shown at upper level in other two assays (ex. Fig. 3 and Fig. 5) and vice versa.
Similarly, the lower 10-15 rankers in one assay were also shown at lower levels in the lists of other two assays or in the pro-oxidant lists of other two assays. Thus, each result obtained from three assays can be useful information on anti-oxidant activities of these plants. However, a few exceptional results, if any, were also found. For example, Crataegus pinnatifida Bunge var. typica Schneider/ Crataegus pinnatifida〈20〉 which was 10th ranker in luminol/Fenton reagent assay (Fig. 1) exhibited pro-oxidant activity in the assays of DCHF/Fenton reagent (Fig. 4) and DCHF/peroxynitrite (Fig. 6). Rosa laevigata Michaux/ Rosa laevigata〈54〉 which had almost no activity (59th ranker) in luminol/Fenton reagent assay (Fig. 1) showed rather strong anti-oxidant activity in DCHF/Fenton reagent (Fig. 3) and DCHF/peroxynitrite assays (Fig. 5). Sangusorba officinalis L./ Sanguisorbae radix〈56〉 was strong anti-oxidant in the assays using luminol/Fenton reagent (Fig. 1) and DCHF/Fenton reagent (Fig. 3) but showed pro-oxidant activity in the DCHF/peroxynitrite assay (Fig. 6). The crude water extracts of the herbs used for the assays contained a variety of substances, which may be the reason for these conflicting results.
One thing to note here is that in all three assay systems, we found that some herbs augmented the radical reactions, i.e. pro-oxidant. We do not know whether this action can occur in vivo and can harm patients in the clinical use of these plants. Although we do no know its meaning or significance now, however, the pro-oxidant activity of these plants may be new information we should pay attention to, particularly in relation to their side effects or toxicities.
In the present study, about 10 herbs in each assay were found to have strong anti-oxidant activity compared to other plants and some of them were overlapped. At present, we do not know how this anti-oxidant action relates to the clinical actions of these plants described in the ascent literatures. The uppermost ranker in each assay was Rubus coreanus Miquel/ Rubus schizostylus, Schisandra chinensis Baillon/ Schizandra chinensis and Terminalia chebula Retzius/ Terminalia chebula, respectively. Rubus coreanus Miquel is known as raspberry. It contains an abundance of sugars, vitamins, minerals, and polyphenols (Bushman et al., 2004; Siriwoharn et al., 2004) and was reported to have anti-inflammatory, antinociceptive, anti-gastropathic and anti-rheumatic effects (Erdemoglu et al., 2003; Nam et al., 2006). Its uses as alcoholic or non-alcoholic beverages have been popularly increased. Based upon its strong anti-oxidant activity, it is highly recommendable to expand its uses. Schisandra chinensis Baillon/ Schizandra chinensis has been used for inflammatory liver diseases and the extract of this plant prevented CCl4-induced liver damage (Chang, 2003), suggesting that its pharmacological effect is related to its anti-oxidant activity. This herb contains schizandrol and its related compounds, which contain phenolic -OH and -OCH3 (Chang, 2003) and possibly another strong anti-oxidant compounds. Thus, it is needed to find new compounds from this herb and also to develop its use in various forms of beverages. Terminalia chebula Retzius/ Terminalia chebula had been prescribed mainly for gastrointestinal disorders such as nausea, vomiting, diarrhea and intestinal distension (Chang, 2003) but nowadays, it does not seem to be prescribed often. Recent studies (Monika et al., 2005) showed that this herb have antibacterial, antidiabetic, antioxidative and radioprotective activities (Gandhi & Nair, 2005; Koteswara & Nammi, 2006; Naik et al, 2005; Rani & Khullar, 2004) and another study reported that it contains anti-oxidant compounds such as gallic acid and quercetin (Nakatani, 2000). Regarding its strong anti-oxidant action, it seems to be worth further developing its use in medicine and food industry.
In the present study, attempts were made first to assess and compare the anti- and pro-oxidant actions of the commonly used herbs in Korean traditional medicine. The results obtained are expected to serve as information for understanding their pharmacological effects, developing new drugs from these herbs, searching natural anti-oxidants or expanding its uses as various forms of beverages.
Footnotes
This work was supported by a grant from the Korean Ministry of Science & Technology through the National Research Laboratory Program for Free Radicals (Grant 2006~2293), by the SRC/ERC program of MOST/KOSEF (#R11-2005-017, Research Center for Women's Disease), by the Brain Korea 21 program, and by a Seoul Science Fellowship.
References
- 1.Bagchi D, Bagdhi M, Hassoun SJ, Stochs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 2.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–485. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Bushman BS, Phillips B, Isbell T, Ou B, Crane JM, Knapp SJ. Chemical composition of craneberry (Rubus spp.) seeds and oils and their antioxidant potential. J Agric Food Chem. 2004;52:7982–7987. doi: 10.1021/jf049149a. [DOI] [PubMed] [Google Scholar]
- 4.Catapano AL, Maggi FM, Tragni E. Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr Opin Cardiol. 2000;15:355–363. doi: 10.1097/00001573-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chang IS. Treatise on Asian herbal Medicines. Vol. 1. Seoul, Republic of Korea: Natural Products Research Institute, Seoul National University publishing department; 2003. p. 2. [Google Scholar]
- 6.Eder K, Flader D, Hirche F, Brandsch C. Excess dietary vitamin E lowers the activities of antioxidative enzymes in erythrocytes of rats fed salmon oil. J Nutr. 2002;132:3400–3404. doi: 10.1093/jn/132.11.3400. [DOI] [PubMed] [Google Scholar]
- 7.Erdemoglu N, Kupeli E, Yesilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2003;89:123–129. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi NM, Nair CK. Radiation protection by Terminalia chebula: some mechanistic aspects. Mol Cell Biochem. 2005;277:43–48. doi: 10.1007/s11010-005-4819-9. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell, Gutteridge Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1:1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 10.Hausladen A, Stamler JS. Nitrosative stress. Meth Enzymol. 1999;300:389–395. doi: 10.1016/s0076-6879(99)00143-3. [DOI] [PubMed] [Google Scholar]
- 11.Jakubowski W, Bartoz G. 2, 7-dichlorofluorescin oxidation and reactive oxygen species: What does it measure? Cell Biol Int. 2000;24:757–760. doi: 10.1006/cbir.2000.0556. [DOI] [PubMed] [Google Scholar]
- 12.Koteswara RN, Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2006;6:17–29. doi: 10.1186/1472-6882-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.1Lee S, Suh I, Kim S. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000a;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- 14.2Lee YM, Kim H, Hong EK, Kang BH, Kim SJ. Water extract of 1:1 mixture of Phellodendron cortex and Aralia cortex has inhibitory effects on oxidative stress in kidney of diabetic rats. J Ethnopharmacol. 2000b;73:429–436. doi: 10.1016/s0378-8741(00)00302-0. [DOI] [PubMed] [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Liu RH. Protective role of phytochemicals in whole foods: implications for chronic disease prevention. Appl Biotechnol Food Sci Policy. 2003;1:39–46. [Google Scholar]
- 17.Monika B, Anurag P, Dhan P. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–291. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- 18.Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mohan H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res. 2005;19:582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 19.Nakatani N. Phenolic antioxidants from herbs and spices. Biofactors. 2000;13:141–146. doi: 10.1002/biof.5520130123. [DOI] [PubMed] [Google Scholar]
- 20.Nam JH, Jung HJ, Choi J, Lee KT, Park HJ. The Anti-gastropathic Anti-rheumatic Effect of Niga-ichigoside F(1) and 23-Hydroxytormentic Acid Isolated from the Unripe Fruits of Rubus coreanus in a Rat Model. Biol Pharm Bull. 2006;29:967–970. doi: 10.1248/bpb.29.967. [DOI] [PubMed] [Google Scholar]
- 21.Rani P, Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytother Res. 2004;18:670–673. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 22.Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J Agric Food Chem. 2004;52:8021–8030. doi: 10.1021/jf048619y. [DOI] [PubMed] [Google Scholar]
- 23.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 24.Zanon FS, Ceriatti M, Rovera LJ, Sabini BA, Ramos T. Search for antiviral activity of certain medicinal plants from Cordoba, Argentina. Rev Latinoam Microbiol. 1999;41:59–62. [PubMed] [Google Scholar]
- 25.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 26.Zhu H, Bannenberg G.L, Moldeus P, Shertzer HG. Oxidation pathways for the intracellular probe 2, 7-dichlorofluorescein. Arch Toxicol. 1994;68:582–587. doi: 10.1007/s002040050118. [DOI] [PubMed] [Google Scholar]
- 27.Zhu YP. Chinese Materia Medica chemistry, pharmacology and applications. Netherlands: Harwood academic publishers, Inc; 1998. pp. 235–245. [Google Scholar]