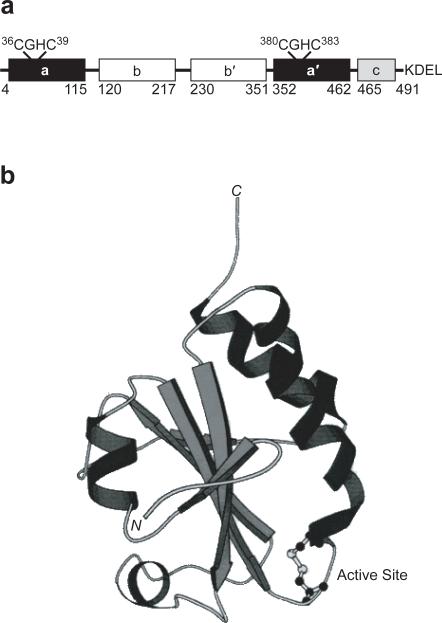
Figure 1.
PDI is composed of four thioredoxin-like domains. a. The a and a′ domains each contain a CGHC active site. The b and b′ domains are likely involved in substrate binding. The c domain is cationic and ends with a C-terminal ER-retention sequence. The numbering system used here is based on the sequence of rat PDI (15). b. The thioredoxin fold of the oxidized a domain of PDI as revealed by NMR spectroscopy (41). The CGHC active site is at the N-terminus of an α helix.