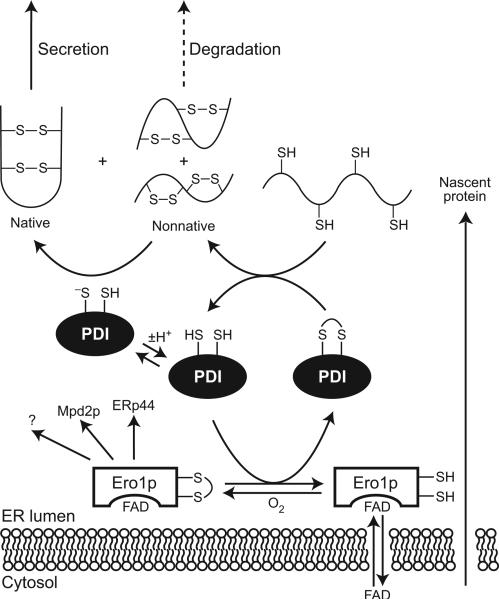
Figure 3.
The pathway of native disulfide formation in the lumen of the ER. FAD-bound Ero1p (83) (and presumably Ero1-Lα and Ero-Lβ in humans) specifically oxidizes PDI as well as ERp44 (human cells), Mpd2p (yeast), and perhaps other proteins (1, 23). Ero1p uses molecular oxygen to reoxidize itself for further folding cycles (84). Oxidized PDI catalyzes the formation of disulfide bonds in newly synthesized proteins; the thiolate form of reduced PDI catalyzes the isomerization of nonnative disulfide bonds (49). Proteins that do not achieve the native state are degraded rather than secreted. For simplicity, only one of the two active site of Ero1p and PDI is shown.