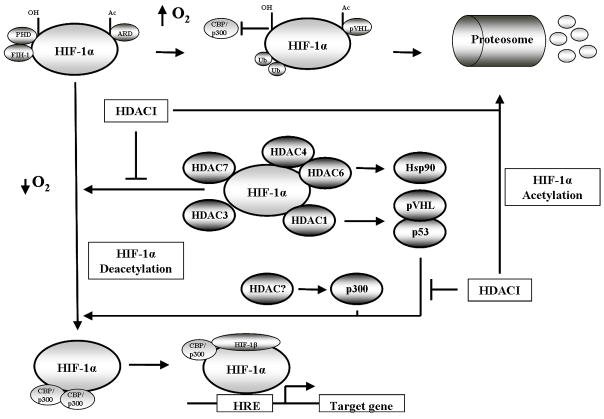
Fig 1.
Schematic cartoon demonstrating the regulation of HIF-1α transcriptional activity. Under normoxic conditions (top row) HIF-1α is hydroxylated, acetylated and bound by the von Hipple-Lindau (pVHL) ubiquitin E3 ligase complex, resulting in polyubiquitination and the proteosomal degradation of HIF-1α. Under hypoxic conditions (bottom row) HIF-1α hydroxylation and acetylation are inhibited due to low oxygen, stabilizing HIF-1α. HIF-1α translocates to the nucleus to bind HIF-1β and recruit CBP/p300 resulting in gene transcription. Hypoxia also induces HDAC expression (middle row) which deacetylates HIF-1α either directly or indirectly to increase HIF-1α transcriptional activity. HDAC inhibition reverses the activity of HDACs resulting in the degradation of HIF-1α.