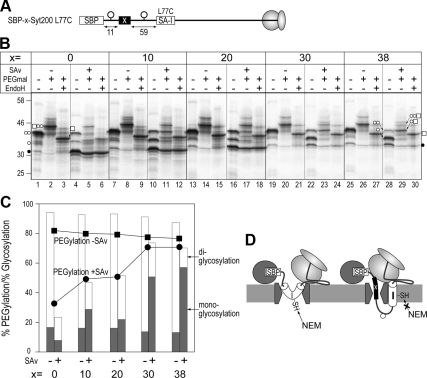
Figure 3.
Migration of the SA-I sequence into the hydrophobic environment synchronizes with partial translocation of the N-domain. (A) The SA-I sequence was separated from the SBP-tag by insertion of a hydrophilic sequence of the lengths indicated by the “x =” row in B. Leu77 within the membrane-embedded region was exchanged for a Cys residue. (B) Molecules with spacer sequences (0, 10, 20, 30, or 38 residues) were synthesized in the absence (−) or presence (+) of streptavidin (SAv) and applied to two-step Cys alkylation and endoglycosidase H (EndoH) treatment, as in the above figures. (C) Monoglycosylated and diglycosylated efficiencies (filled and open columns, respectively) in the cases with (+) or without (−) SAv were quantified from PEGmal (−) lanes in B. PEGylation efficiencies in the presence (•) or absence (■) of SAv were also quantified from the EndoH + lanes. (D) As the spacer (x) was elongated, the N-domain partially reached the lumenal space even when the N-terminal SBP-tag was trapped in the cytosol. In this situation, the SA-I sequence formed the TM orientation and migrated into the hydrophobic interior of the membrane.