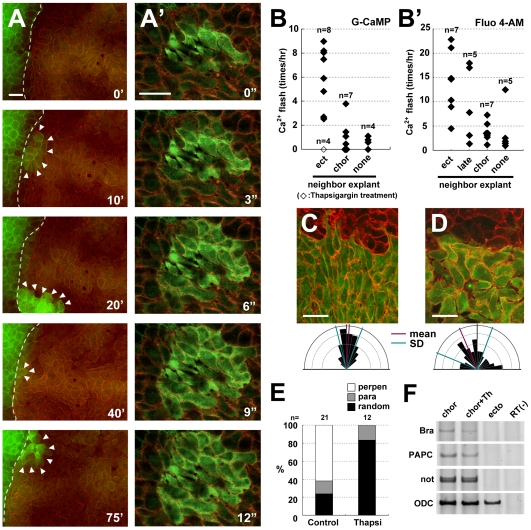
Figure 1. Increased intracellular calcium is induced in chordamesodermal cells by tissue attachment and is required for cell polarization.
(A) Time-lapse analysis of the calcium dynamics in a heterogeneous conjugation assay taken by each 40 seconds. The right side of the explant (the cytoplasmic membranes were marked by RFP (red), co-injected with G-CaMP (green)) was chordamesoderm induced by the overexpression of nodal mRNA (150 pg), and the left side (the cytoplasmic membranes marked by GFP (green)) was ectoderm. The dotted line shows the boundary between the two explants, and the arrowheads indicate G-CaMP signals. (A′) The time-lapse images showing the calcium propagation indicated by G-CaMP in the chordamesoderm tissue taken by each 3 seconds. (B, B′) The frequency of calcium flashes in chordamesodermal tissues conjugated with heterogeneous neighboring explants, indicated by G-CaMP (B) or Fluo 4-AM (B′). Each diamonds show the average number of G-CaMP or Fluo-4 signals observed per hour in 10 cells at the boundary in each explants, and the open square in B shows the frequency under treatment with thapsigargin. (ect: ectoderm, chor: chordamesoderm, late: lateral mesoderm, none: no neighboring explant) (C) Perpendicular alignment of chordamesodermal cells in the conjugation assay with ectoderm. The upper part marked by membrane-RFP was ectoderm, and the lower part marked by GFP cytoplasm and membrane-RFP was chordamesoderm. The directions of the long axis of each cell were indicated by a rose diagram. (D) Abnormal cell alignment caused by thapsigargin treatment. The cells were aligned randomly compared with C. (E) Proportion of alignment types in relation to the border in the chordamesoderm shown in (C) and in the thapsigargin-treated sample shown in (D). (para: parallel, perpen: perpendicular) (F) RT-PCR analysis of mesodermal induction by the overexpression of nodal mRNA. Thapsigargin treatment did not affect the mesodermal induction. (bra: brachyury, Th: Thapshigargin) (bar: 50 µm).