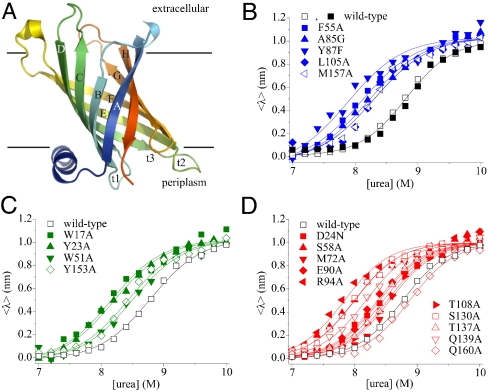
Fig. 1.
(A) Cartoon representation of PagP [Protein Data Bank (PDB) ID code 1THQ (19); W. L. DeLano, http://www.pymol.org (2002)]; β-strands are labeled A–H; periplasmic turns t1–3. (B) Equilibrium refolding (▪) and unfolding (□) of wild-type PagP. The unfolding transitions of PagP variants bearing mutations in residues in (B) the hydrophobic surface, (C) the aromatic girdles, and (D) the barrel interior are also shown. For these variants residues mutated that are located in the N-terminal half of the protein sequence are indicated by closed symbols, whereas residues in the C-terminal half of the protein sequence are shown as open symbols. Solid lines are global fits to a two-state mechanism yielding a common MUN = 6.86 ± 0.20 kJ/mol/M. All experiments were performed using 0.4 μM PagP in diC12∶0PC-liposomes at an LPR of 3200∶1 in 50 mM sodium phosphate buffer pH 8 at 25 °C.