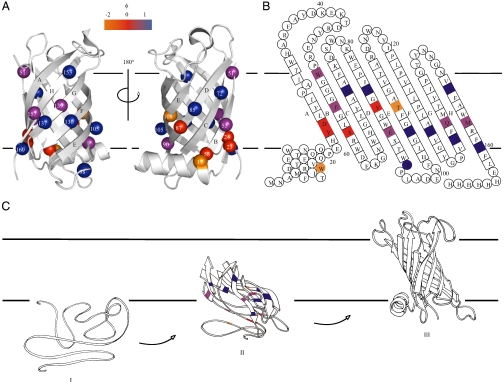
Fig. 4.
(A) Cartoon representation of PagP [PDB ID code 1THQ (19); W. L. DeLano, http://www.pymol.org (2002)] with ΦF-values mapped onto the native structure. (B) Topology map of PagP highlighting the ΦF-values of mutated residues. (C) Proposed folding mechanism of PagP into membranes: unfolded, but membrane-associated PagP (I); tilted insertion of the transition-state ensemble (II); and assembly of the helical clamp to yield native PagP (III). Note that the periplasmic halves of strands A, B, C, and D are depicted as loops to depict the low ΦF-values of side-chains in this region of the transition-state structure. In A–C, ΦF > 0.5 are in blue, 0.3 ≤ ΦF ≤ 0.5 are in purple, ΦF < 0.3 are in red, and ΦF < 0 are in orange. Residues 51, 72, 108, 137, and 160 gave 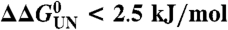 [the cut-off generally accepted to yield highly reliable ΦF-values (41)].
[the cut-off generally accepted to yield highly reliable ΦF-values (41)].