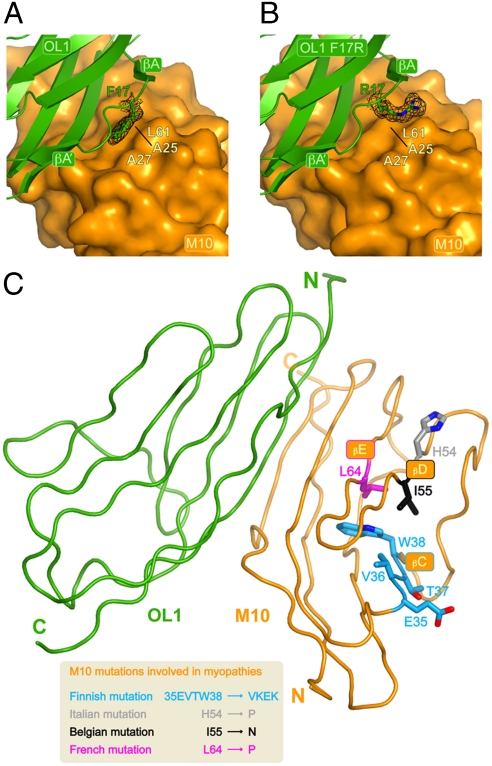
Fig. 2.
(A) Close-up on the OL1 F17 residue with M10 shown as surface representation. The OL1 F17 side chain is buried and stabilized by M10 residues A25, A27 and L61. 2mFo-DFc electron density for OL1 F17 is shown in black at the 1.3σ contour level. (B) Closeup of the OL1 R17 residue in the M10-OL1 F17R complex engineered to partly mimic the M10-O1 complex. OL1 R17 points its charged side-chain towards the solvent. 2mFo-DFc electron density for OL1 R17 is shown in black at the 1.3σ contour level. The orientation is the same as in A. (C) View of the M10-OL1 complex highlighting the position of the M10 residues involved human cardiomyopathies as a result of four different (Finnish, Belgian, French and Italian) genetic mutations (see main text). Amino acid changes resulting from the different mutations are shown in the inset.