Abstract
Cognitive control, the ability to voluntarily guide our behavior, continues to improve throughout adolescence. Below we review the literature on age-related changes in brain function related to response inhibition and working memory, which support cognitive control. Findings from studies using functional magnetic imaging (fMRI) indicate that processing errors, sustaining a cognitive control state, and reaching adult levels of precision, persist through adolescence. Developmental changes in patterns of brain function suggest that core regions of the circuitry underlying cognitive control are on-line early in development. However, age-related changes in localized processes across the brain and in establishing long range connections that support top-down modulation of behavior may support more effective neural processing for optimal mature executive function. While great progress has been made in understanding the age-related changes in brain processes underlying cognitive development, there are still important challenges in developmental neuroimaging methods and the interpretation of data that need to be addressed.
Keywords: response inhibition, working memory, prefrontal cortex, saccades, executive function, blood oxygen level dependent (BOLD), distributed circuitry, ventrolateral prefrontal cortex, adolescence, cognitive control, brain development
I. Introduction
Adolescence is a period when many aspects of decision-making appear adult-like, yet adolescents’ decisions are inconsistent, and this often leads to suboptimal or even dangerous behavior. The ability to voluntarily guide behavior in a goal-directed fashion is essential to mature decision making. Cognitive control and executive function are terms used to describe the processes that allow us to voluntarily guide our behavior. While adolescents can exhibit sophisticated voluntary behavior, the ability to do so consistently continues to improve during adolescence, making cognitive control a particularly useful model for investigating the vulnerabilities of this period. Characterizing the brain circuitry underlying immaturities in cognitive control can elucidate the important aspects of the neural basis of decision making in adolescence. This paper reviews how typical adolescents differ from adults on two fundamental aspects of cognitive control - response inhibition and working memory (Fuster, 1997b). Response inhibition refers to the ability to voluntarily select a task-appropriate, goal-directed response while suppressing a more compelling but task-inappropriate response. Working memory refers to the mental ‘sketch pad’ that allows us to retain relevant information on-line in order to make a planned, goal-directed response. While these processes work together to support goal driven behavior (Miller & Cohen, 2001), they can be considered independently to characterize specific profiles of cognitive development (Luna, Garver, Urban, Lazar, & Sweeney, 2004; Demetriou, Christou, Spanoudis, & Platsidou, 2002a).
Major psychopathology can often emerge or intensify during adolescence, indicating that there are vulnerabilities inherent to this developmental transition that may contribute to mental disorders. Additionally, cognitive control is usually compromised in major psychopathology (Luna & Sweeney, 2004a; Sweeney, Takarae, Macmillan, Luna, & Minshew, 2004). This suggests that investigating neurocognitive development can serve as a way to probe the integrity of complex brain circuitries associated with psychopathology. For example, if the maturation of long-range connectivity in the brain proves to be important for the late developmental improvements in cognitive control, these processes may be compromised in psychopathology. Additionally, normative adolescence is also characterized by a peak in sensation-seeking that can lead to risk-taking behavior that undermines survival (Chambers, Taylor, & Petenza, 2003; Spear, 2000). Delineating the normative processes that underlie the improvement in cognitive control from adolescence to adulthood provides a template for understanding the neurobehavioral basis of both psychopathology and risk-taking behavior.
Given that behavior is the result of both environmental and biological influences, the ability to assess their association is optimal for characterizing developmental change. Neuroimaging methods provide one approach to investigate concurrent changes in brain and behavior. Functional Magnetic Resonance Imaging (fMRI) is particularly appropriate for developmental studies because it is non-invasive and allows the assessment of brain function relevant for a specific behavior. However, the interpretation of developmental neuroimaging results differs from that of adult neuroimaging studies, because results from younger individuals must be considered in the context of brain function in adulthood. That is, we identify immaturities in brain function in young individuals by comparing them to results in mature adult populations. In this paper, we review the literature characterizing age-related changes in brain function underlying cognitive control of behavior through adolescence. We consider studies that have focused on voluntary response inhibition and those that measure changes in response to working memory demands. We end by discussing methodological issues that are specific to interpreting developmental fMRI results.
The Development of Cognitive Control
Voluntary planned behavior requires the ability to retain online the goal of the response (working memory), to plan and prepare the response, and the ability to suppress task irrelevant responses in order to make a task appropriate response (response inhibition). In this section, we review how basic components of cognitive control (response inhibition and working memory) change during childhood and adolescence, focusing on neuroimaging studies examining changes in brain function.
Response inhibition
Response inhibition is central to the voluntary control of behavior, providing the flexibility needed for behavior to be guided by a task goal (Miller & Cohen, 2001; Davidson, Amso, Anderson, & Diamond, 2006; Fuster, 1997b). As in real life, what is being inhibited varies across experimental tasks – it may be extremely prepotent, such as reflexive responses to external stimuli (e.g., an-tisaccade task), or a learned automatic response (e.g., go-no-go task). The most frequently used tasks in clude the go-no-go, flanker, stop signal, antisaccade, and Stroop tasks. The go-no-go task requires that subjects suppress a repeated response that was established during previous trials (pressing a button for any letter but “x”), while the Stroop and flanker tasks require suppression of well-entrenched responses (color words, arrows), and the antisaccade and stop signal task require the suppression of an inherent motor response (to not look at a light, to stop a button press midway). While these tasks tap into different aspects of inhibition (Kok, 1999), they all require top down modulation of behavior.
Infants are able to suppress attention to a distracter stimulus in order to produce the most task appropriate response (Diamond & Goldman-Rakic, 1989; Amso & Johnson, 2005; Bell & Fox, 1992). In addition, EEG evidence indicates that infants are already using frontal systems to support these inhibitory responses (Bell & Fox, 1992), despite the protracted development of these regions (Gogtay, Giedd, Lusk, Hayashi, Greenstein et al., 2004). However, there are continued improvements throughout childhood, consistent across different inhibitory tasks, in the rate of inhibitory responses (percent of correct responses).This continued development throughout childhood is evident on a number of behavioral tasks such as the antisaccade (Fischer, Biscaldi, & Gezeck, 1997; Munoz, Broughton, Goldring, & Armstrong, 1998; Fukushima, Hatta, & Fukushima, 2000; Klein & Foerster, 2001; Luna, Garver, Urban, Lazar, & Sweeney, 2004), Stroop (Tipper, Bourque, Anderson, & Brehaut, 1989; Wise, Sutton, & Gibbons, 1975), go-no-go (Levin, Culhane, Hartmann, Evankovich, & Mattson, 1991), stop-signal (Williams, Ponesse, Schachar, Logan, & Tannock, 1999; Ridderinkhof, Band, & Logan, 1999; Greenberg & Waldman, 1993), and Flanker tasks (Ridderinkhof, van der Molen, Band, & Bashore, 1997). In summary, behavioral studies of inhibitory performance through childhood indicate that what generally improves is the rate of correct inhibitory responses but not the ability to generate a correct inhibitory response (Williams, Ponesse, Schachar, Logan, & Tannock, 1999; Wise, Sutton, & Gibbons, 1975; Ridderinkhof, Band, & Logan, 1999; Bedard, Nichols, Barbosa, Schachar, Logan et al., 2002; Van den Wildenberg & van der Molen, 2004; Luna, Garver, Urban, Lazar, & Sweeney, 2004).
Our studies using the antisaccade task also show a prolonged developmental improvement in the rate of correct inhibitory responses, consistent with previous studies using this paradigm (Fischer, Biscaldi, & Gezeck, 1997; Munoz, Broughton, Goldring, & Armstrong, 1998; Fukushima, Hatta, & Fukushima, 2000; Klein & Foerster, 2001; Luna, Garver, Urban, Lazar, & Sweeney, 2004). In the antisaccade task, subjects are asked to look away from a suddenly appearing target in an unpredictable location, and instead to look to its mirror location in the opposite direction. Saccading to a visual stimulus is a prepotent response, since the visual, oculomotor, and attention systems are geared primarily to identifying stimuli in the environment. The ability to suppress such a prepotent response requires top-down modulation from frontoparietal regions to subcortical regions, in order to engage preparatory processes that are able to stop a reflexive response (Everling & Fischer, 1998). Results reliably indicate improvements from early childhood, when approximately 50% of trials are errors, to adulthood, when only 10–20% are errors (Fischer, Biscaldi, & Gezeck, 1997; Munoz, Broughton, Goldring, & Armstrong, 1998; Fukushima, Hatta, & Fukushima, 2000; Klein & Foerster, 2001; Luna, Garver, Urban, Lazar, & Sweeney, 2004). Developmental improvement continues until mid to late adolescence, at which point the rate of correct inhibitory responses reaches approximately adult levels.
We propose that these results indicate that the neural mechanisms that support inhibition of a response, as an isolated event, are available early in development but may be slow and less efficient than the adult system. The ability to produce a high rate of correct inhibitory responses across a number of trials by sustained inhibitory control, continues to improve through adolescence (See Below). Thus, this ability to produce a continuous high rate of correct responses reflects a mature system that has flexibility and ease in engaging executive systems, including setting a response state in place, while the immature system may be limited in this ability.
Response inhibition is supported by a widely distributed circuitry of which ventrolateral prefrontal cortex (VLPFC) is believed to play a primary role. VLPFC has connections to other prefrontal regions and sensory and motor regions and has been found to support the selection of the particular behavior to be inhibited (Sakagami & Pan, 2007). Moreover, evidence of late structural changes in prefrontal cortex (PFC) (Huttenlocher, 1990) make this region particularly important for understanding cognitive development. Therefore, most developmental fMRI studies of response inhibition have focused exclusively on the PFC. Across studies, findings indicate that the inferior frontal gyrus (IFG) (BA45/46) and premotor regions (BA46) increase in activation with age (Rubia, Overmeyer, Taylor, Brammer, Williams et al., 2000; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). Activation of these regions have been found in developmental studies across inhibitory tasks such as the go-no-go (Rubia, Smith, Woolley, Nosarti, Heyman et al., 2006; Tamm, Menon, & Reiss, 2002), flankers (Bunge, Dudukovic N.M., Thomason, Vaidya C.J., & Gabrieli J.D.E., 2002), stop tasks (Rubia, Smith, Taylor, & Brammer, 2007), Stroop (Adleman, Menon, Blasey, White, Warsofsky et al., 2002; Marsh, Zhu, Schultz, Quackenbush, Royal et al., 2006b), and antisaccade tasks (Luna & Sweeney, 2001), with both equivalent performance or with better performance in adults.
However these same studies, as well as others, also find age-related decreases in the activation of other prefrontal regions including IFG and medial frontal gyri (MFG). For instance, a go-no-go study on 8- to 20-year-olds showed both increases in activation in the medial frontal gyrus as well and decreases in the IFG (Tamm, Menon, & Reiss, 2002). These authors hypothesized that age-related increases in MFG activation reflect improved inhibitory processes with age, related to limitations in the processing of information in this region in younger individuals, while age-related decreases in IFG activation reflect the decreased effort required to exert inhibitory control with age. Our own studies have shown that during adolescence (13–17 years of age) there is increased recruitment of dorsolateral prefrontal cortex (DLPFC) more so than in either adults or children (8–12 years of age), reflecting that adolescents show adult-like performance but that this achievement requires greater effort (Luna, Thulborn, Munoz, Merriam, Garver et al., 2001). However, there are alternate interpretations of the PFC activation. Broca’s area (BA 45) in IFG has also been considered as part of VLPFC and could possibly be supporting verbal strategies (leading to developmental changes since use of these sort of strategies increase with age), while the inferior aspect of the precentral sulcus, which is part of the frontal eye field (FEF - BA6) may provide preparatory information to attenuate reflexive responses (Everling & Munoz, 2000).
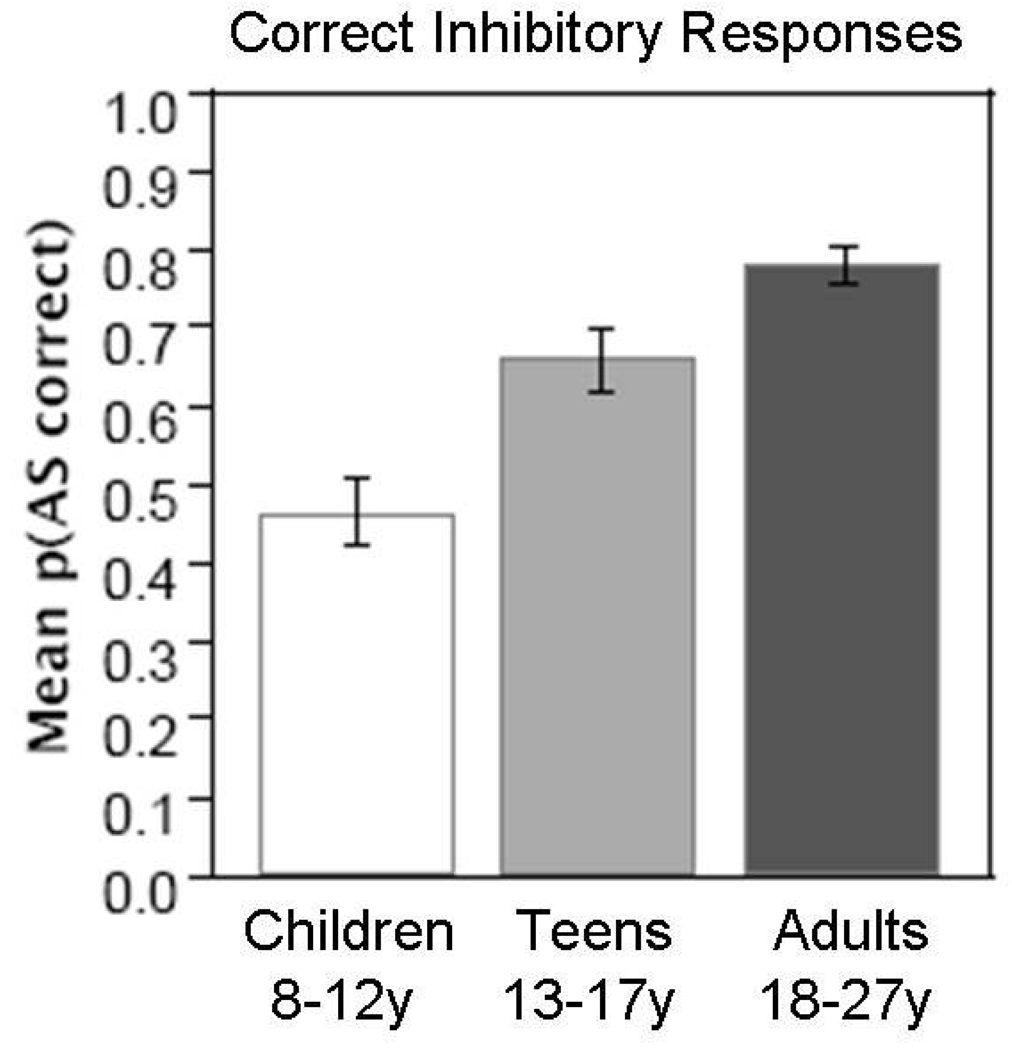
Although the PFC has a protracted development, so do other neocortical regions including parietal and temporal regions (Gogtay, Giedd, Lusk, Hayashi, Greenstein et al., 2004). Our event-related studies investigated developmental changes during correct trials as a model to identify changes in brain function underlying equivalent performance. Behavior in the scanner showed that adolescents (13–17 years of age) performed better than children (8–12 years of age) but did not reach adult levels (18–27 years of age) in rate of correct inhibitory responses (See Figure 1). Activation results indicated that across childhood, adolescence, and adulthood, a comparable network is recruited including canonical eye movement regions, ACC and right DLPFC (Velanova, Wheeler, & Luna, 2008) (See Figure 2). Group comparisons (which are not evident in Figure 2) showed that, while adolescents displayed adult-like levels of activation in these regions, children showed increased activity in DLPFC suggesting increased effort to perform the task. Adolescents and adults instead showed greater reliance on posterior visual and parietal regions compared to children. While adolescents showed activation equivalent to adults during correct trials, they produced significantly more errors than adults. These results suggest that while rate of performance is immature in adolescence, correct individual inhibitory responses are supported by a mature circuitry that includes less reliance on prefrontal regions.
Figure 1.
Data from Velanova et al., 2008. Mean percent of correct inhibitory responses (± 1 SEM) for children (8–12y), adolescents (13–17y), and adults (18–27y) generated while performing the antisaccade task inside the scanner. Reprinted with permission from Cerebral Cortex.
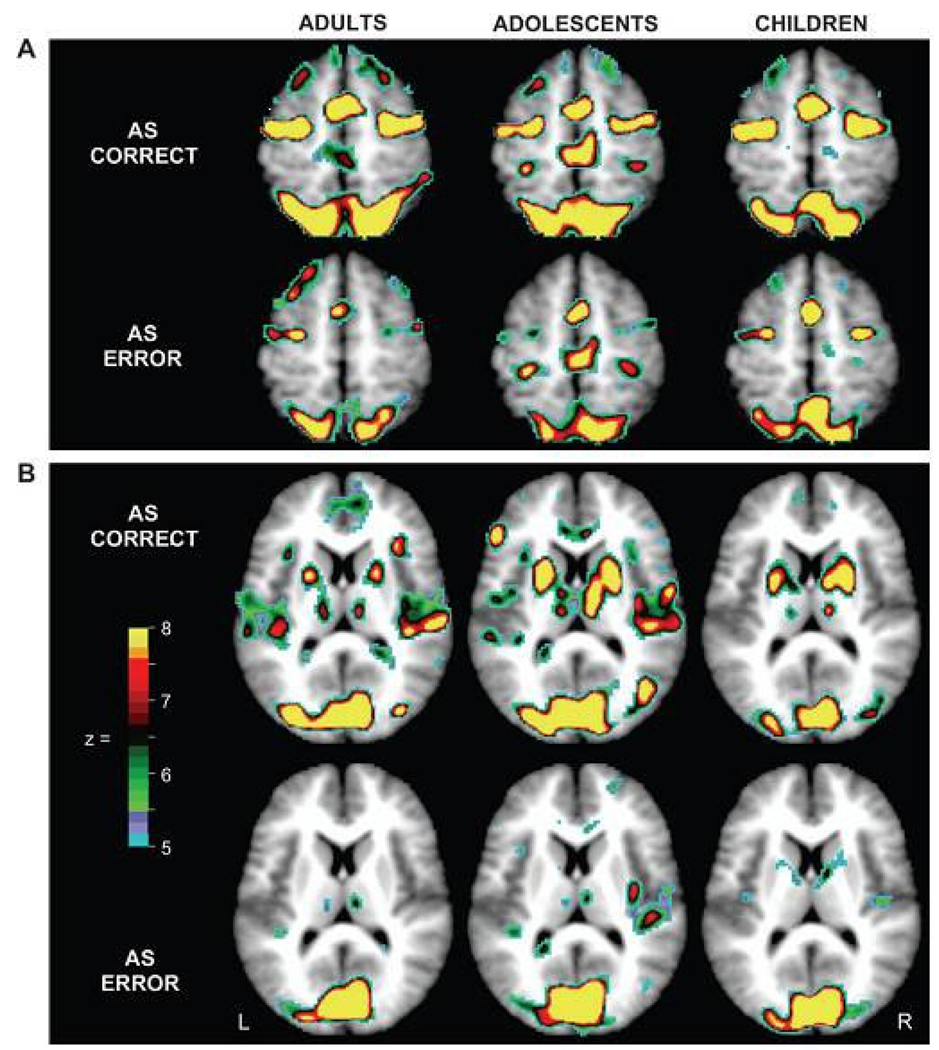
Figure 2.
From Velanova et al., 2008. Voxels showing significant statistical activity change for correctly and incorrectly performed AS trials (i.e., correct and error trials) in adults, adolescents and children. (A) Horizontal sections show differing activity for correct and error trials, though similar distributions and levels of activity across age groups in supplementary motor area (SMA)/preSMA, frontal eye field (FEF), and posterior parietal cortex (PPC). (B) Horizontal sections show increased activity in putamen for correct trials relative to error trials in all age groups. Note that because maps are based on ANOVA, the direction of effects is not represented. Reprinted with permission from Cerebral Cortex.
In conjunction with changes in prefrontal function, studies indicate age-related changes in widespread regions outside of PFC including parietal, temporal, subcortical and cerebellar regions. These changes are associated with improvements in inhibitory control (Rubia, Smith, Woolley, Nosarti, Heyman et al., 2006; Luna, Thulborn, Munoz, Merriam, Garver et al., 2001; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). fMRI studies suggest that age-related improvements in the integration of frontal regions with other areas, including frontal-striatal-thalamic and frontal-cerebellar circuits, support the development of inhibitory control and correlate with better performance on inhibitory tasks (Rubia, Smith, Woolley, Nosarti, Heyman et al., 2006; Rubia, Smith, Taylor, & Brammer, 2007). For instance, a recent study examining functional connectivity using independent component analyses during a go/no-go task found developmental changes in network activity from adolescence (11–17 years of age) to adulthood (18–37 years of age) (Stevens, Kiehl, Pearlson, & Calhoun, 2007). These authors report age-related increases in activation of a frontal-parietal network, as well as increased mutual engagement of a frontal-striatal-thalamic network. This suggests that network activity may become more specialized and integrated throughout adolescence. Adolescents also showed greater activity in right VLPFC, presumably compensating for immaturities in the integration of these circuits. This, along with the fact that engagement of the frontal-parietal circuit was related to performance in adolescents but not adults, suggests that good performance required more effort and monitoring – and thus more PFC activation – in adolescents than in adults. In addition to the coordinated activity in circuits including parietal, cerebellar and subcortical systems, mature performance may also reflect improved circuitry that includes regions in anterior cingulate cortex (ACC) involved in error monitoring, as well as oculomotor brain regions related to improved ‘response state’, both of which are discussed below.
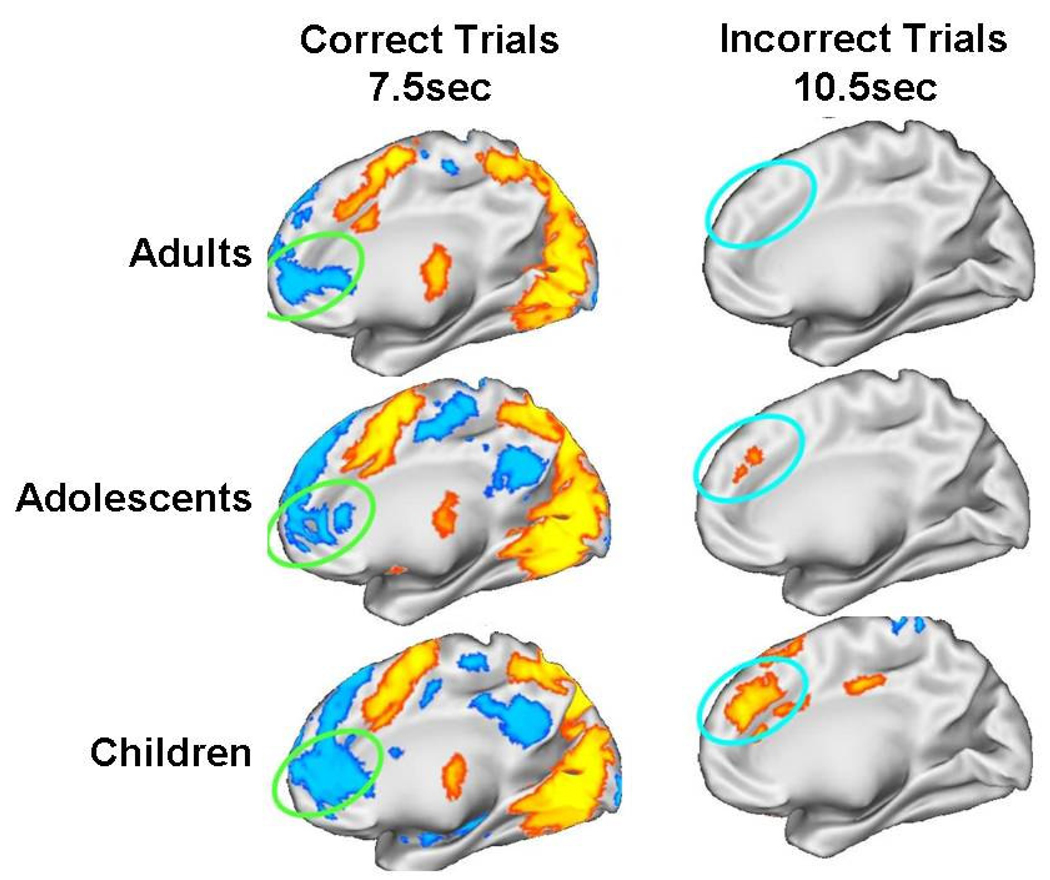
The ability to detect performance errors and monitor performance is crucial to inhibitory control and is supported by a well-delineated neural circuitry of which ACC is central (Carter, Braver, Barch, Botvinick, Noll et al., 1998; Braver, Barch, Gray, Molfese, & Snyder, 2001). Initial studies have found that recruitment of ACC, like PFC, increases with development. Rubia et al. (2007) found that when inhibitory performance was equated across childhood (10–17 years of age) to adulthood (18–47 years of age) by adapting the task by skill level, adults demonstrated increased recruitment of ACC and PFC compared to children. This result suggests that ACC may continue to mature into adulthood, supporting more effective performance monitoring. A neuroimaging study in adults examined the evolution of ACC participation in antisaccade performance and found an early negativity associated with correct responses in rostral ACC and increased activation later in the trial during error commission in dorsal ACC (Polli, Barton, Cain, Thakkar, Rauch et al., 2005), the putative regions for error evaluation and adjustment (Carter, Braver, Barch, Botvinick, Noll et al., 1998; Braver, Barch, Gray, Molfese, & Snyder, 2001). To make a correct inhibitory response, it is necessary to initially suppress activity in rostral ACC, which is believed to support “default-mode” processing, which is engaged when an individual is not focused on a task (See Below). During error commission there is a later recruitment of dorsal ACC, which is believed to integrate error information with planning for upcoming responses (Polli, Barton, Cain, Thakkar, Rauch et al., 2005). Results indicate that, while all age groups showed deactivation of rostral ACC during correct trials, only adults showed robust recruitment of dorsal ACC during inhibitory errors (Figure 3). This pattern suggests that the ability to de-activate the default mode for inhibitory control is on-line by childhood, but error monitoring continues to mature through adolescence. It is important to determine whether performance monitoring shows protracted development and hence accounts for developmental improvements in response inhibition (Velanova, Wheeler, & Luna, 2008). The rate of corrective responses after an error was equivalent across ages indicating that the ability to detect an error is mature. However, the brain processes supporting performance evaluation and adjustment, which are needed to avoid future errors, are still immature.
Figure 3.
Adapted from Velanova et al., 2008. Findings of dissociable medFG/rACC and dACC activity across time for correct and error AS trials in each age group. Statistical activation maps displayed on the partially inflated medial cortical surface of the right hemisphere for correctly performed AS trials and incorrectly performed trial minus correct trials. Blue colors indicate regions that showed decreased activation while yellow/orange colors indicate regions that show increased activation. Reprinted with permission from Cerebral Cortex.
The ability to establish a response set, that is, to sustain focused attention while performing a task, may be a crucial element in the maturation of inhibitory control. It may also have an independent developmental trajectory from the ability to correctly inhibit responses on any given trial. The attention literature has referred to this process as the establishment of a task-related state that allows for the executive organization and control of the processes guiding cognitive events (Logan & Gordon, 2001). A distinct circuitry including dorsal ACC, medial PFC, and bilateral anterior insula has been found to be recruited across different tasks (e.g. verb generation, matching, motor timing) when maintaining a task-set compared to periods of rest fixation (Dosenbach, Visscher, Palmer, Miezin, Wenger et al., 2006). Developmental changes in the recruitment of this circuitry may be particularly important since, across studies, developmental improvements are evident as increases in the percent of correct inhibitory responses, and not the ability to make a single inhibitory response. The ability to retain a cognitive state of performance such as inhibitory control may support the ability to generate a high percentage of correct inhibitory responses, as is evident in adulthood. Therefore, developmental improvements in inhibitory control may be supported primarily by the ability to establish an inhibitory response state and the brain systems supporting this particular ability. This rationale suggests that what characterizes development through adolescence may not be the emergence of a new cognitive ability (inhibitory control) but the ability to use this tool in a flexible and consistent fashion by effectively establishing a response state. This possibility implies that the circuitry that uniquely supports response state (Dosenbach, Visscher, Palmer, Miezin, Wenger et al., 2006) is immature in adolescence. Consistent with this possibility, neuroimaging findings indicate that the circuitry supporting response state shows a protracted development through adolescence (Fair, Dosenbach, Church, Cohen, Brahmbhatt et al., 2007) (See more below).
Working Memory
Voluntary responses require the ability to maintain a representation of the rules that will guide behavior in working memory (Baddeley, 1986). Much like response inhibition, working memory is a central component of executive function (Miller & Cohen, 2001; Fuster, 1997b) and has a protracted developmental trajectory through adolescence (Luna, Garver, Urban, Lazar, & Sweeney, 2004; Demetriou, Christou, Spanoudis, & Platsidou, 2002a). Studies have shown that at both the behavioral and neural levels, fundamental working memory skills and their neural substrates are established by childhood and even infancy (Diamond, Towle, & Boyer, 1994). This finding indicates that, similar to response inhibition, the mechanisms underlying the ability to hold information online develops early, but that the processes underlying the fidelity of the representation kept in working memory, improves through adolescence (Luna & Sweeney, 2004b).
Across a range of verbal, numerical, and spatial working memory tasks, performance has been found to improve throughout childhood (Luna, Garver, Urban, Lazar, & Sweeney, 2004; Demetriou, Christou, Spanoudis, & Platsidou, 2002a; Luciana, Conklin, Hooper, & Yarger, 2005; van Leijenhorst, Crone, & van der Molen, 2007). Phonological working memory tasks however appear to mature earlier than spatial working memory performance, which continues to improve through adolescence (Demetriou, Christou, Spanoudis, & Platsidou, 2002a). Because verbal strategies (due to better verbal comprehension or learned strategies that use verbal processing) could undermine the ability to detect development specific to working memory, developmental studies often use spatial working memory tasks (i.e., nonsense shape or object location). These spatial working memory tasks, reviewed in the next section, show that, while children can identify the general location of a previously presented cue (Luciana, Conklin, Hooper, & Yarger, 2005; van Leijenhorst, Crone, & van der Molen, 2007), the precision of the response continues to improve into adulthood (Luna, Garver, Urban, Lazar, & Sweeney, 2004).
The oculomotor delayed response task (ODR) (also known as the memory-guided saccade task) is a classic spatial working memory task used in single-cell recording studies in monkeys to characterize the neural activity underlying working memory (Hikosaka & Wurtz, 1983; Funahashi, Inoue, & Kubota, 1997). In this task, subjects make a voluntary eye movement to the location where they remember having previously seen a target, after a varying delay period during which they maintain a central fixation stimulus. When the fixation is extinguished, participants must saccade to the remembered location. This response usually involves two or more eye movements. The first saccade, which approximates the target location, is driven both by processes supporting voluntary responses (namely, directing the eyes to move with no visual guidance) and the working memory representation of target location. Subsequent, smaller corrective eye movements are then made, driven predominantly by the working memory representation and error/performance monitoring processes. In a study examining 8 to 30 year olds, we found that the accuracy of the initial saccade became adult like by approximately 15 years of age. However, the last corrective saccade that enhanced the precision of the final response continued to improve into the early twenties (Luna, Garver, Urban, Lazar, & Sweeney, 2004). The last saccadic response may reflect precision in spatial mapping and error monitoring. These results suggest that although the ability to initiate a voluntary response guided by working memory reaches maturity in adolescence, corrective responses that increase the precision of the response continue to mature into early adulthood. This pattern was evident across different delay periods, including short delays of one second, suggesting that maintenance processes alone do not account for developmental improvements in accuracy. Instead, processes underlying spatial precision (e.g., recruiting brain regions that have a greater spatial resolution) during encoding may also improve over development, along with maintenance. These results are consistent with other spatial working memory tasks, such as the self-ordered search tasks, where participants must remember spatial locations sequentially within a trial to guide responses. Results from these tasks also show developmental improvements in working memory processes into early adulthood (DeLuca, Wood, Anderson, Bucanan, Proffitt et al., 2003; Luciana & Nelson, 2002; Demetriou, Christou, Spanoudis, & Platsidou, 2002b; Luciana, Conklin, Hooper, & Yarger, 2005).
Processes separate from central working memory can also limit performance, including interference from distracting stimuli and a failure to use strategies. Due to immaturities in inhibition, children have more interference from distracters than adults, undermining the ability to show mature working memory performance in tasks that present competing stimuli during the delay period (Bjorklund & Harnishfeger, 1990; Dempster, 1981). Adults are also more likely to use strategies such as verbal rehearsal or using associations between to-be-remembered items during working memory maintenance than are adolescents (van Leijenhorst, Crone, & van der Molen, 2007; Cowan, Saults, & Morey, 2006). These strategies enhance performance by using systems in addition to working memory, such as long term memory, to assist in task performance. An advantage of the oculomotor delayed response task is that, typically, no interfering tasks are presented during the delay period, limiting the response inhibition requirements and more effectively isolating the working memory component of these tasks. Consistently, results indicate that although children can guide their behavior by instruction held in working memory, their responses are less accurate than those of adults. What continues to improve into adolescence is the ability to be precise, to control distraction, and to monitor performance, resulting in more exact and adaptable working memory. That is, working memory is on-line early in development but processes related to precision of the maintained representation continue to improve with age.
Similar to response inhibition, a widely-distributed circuitry is known to underlie mature working memory including VLPFC, DLPFC, medial prefrontal regions, the striatum, posterior parietal cortex, the middle temporal gyrus, and the cerebellum (Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998; Curtis, Rao, & D'Esposito, 2004; D'Esposito, Postle, Ballard, & Lease, 1999; Fuster, 1997a; Petit, Courtney, Ungerleider, & Haxby, 1998; Postle, Berger, Taich, & D'Esposito, 2000; Sweeney, Luna, Berman, McCurtain, Strojwas et al., 1996; Thomas, King, Franzen, Welsh, Berkowitz et al., 1999). However, the main focus of developmental research, much like response inhibition, has been on the role of prefrontal systems on working memory. fMRI studies have consistently shown that prefrontal systems are engaged in working memory processes as early as eight years of age but the magnitude of engagement varies with age (Casey, Cohen, Jezzard, Turner, Noll et al., 1995). Prefrontal regions undoubtedly play a role in central aspects of working memory but many studies indicate that other regions, mainly parietal areas, also play an important role in spatial working memory tasks and change over development. Prefrontal areas may support general executive aspects of working memory such as the manipulation of information, while parietal regions may support the mnemonic processes underlying working memory (Postle, Ferrarelli, Hamidi, Feredoes, Massimini et al., 2006; D'Esposito, Postle, Ballard, & Lease, 1999; Geier, Garver, & Luna, 2007a).
fMRI studies using simple working memory tasks such as the n-back task, where responses depend on on-line maintenance of the arrangement of previous cues, show that children recruit similar prefrontal-parietal networks as adults (Thomas, King, Franzen, Welsh, Berkowitz et al., 1999; Nelson, Monk, Lin, Carver, Thomas et al., 2000). However, studies using tasks requiring more complex manipulation and monitoring of information find that children recruit different regions compared to adults (Ciesielski, Lesnik, Savoy, Grant, & Ahlfors, 2006). Age-related changes in brain activity (mainly increases with age) appear to reflect immaturities in the ability to manipulate information in working memory (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006), to generate an accurate response (Scherf, Sweeney, & Luna, 2006; Klingberg, Forssberg, & Westerberg, 2002), and to suppress distractors (Olesen, Macoveanu, Tegner, & Klingberg, 2007).
To investigate neural level changes with development, we performed an ODR task (described earlier) with varying delays in children (10–13 years of age), adolescents (14–17 years of age), and adults (18–30 years of age) in an fMRI study (Scherf, Sweeney, & Luna, 2006). In accord with other working memory studies, DLPFC was recruited across all three age groups. However, similar to our results with response inhibition, the magnitude of right DLPFC participation followed an inverted “U” shaped curve, peaking in adolescence. These results may be due to the fact that children do not perform at adolescent or adults levels, which may be due to immaturities in the cognitive control executive processes related to prefrontal systems. Children’s inferior performance was accompanied by increased reliance on basal ganglia, and insula whereas adults showed a more widely distributed circuitry that included temporal regions. Adolescents performed similarly to adults but may have exerted greater effort, reflected in the increased recruitment of DLPFC. These results are supported by studies of verbal working memory indicating that adults recruit multiple regions across frontal and also parietal areas and that this recruitment increases systematically with cognitive load (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; O'Hare, Lu, Houston, Bookheimer, & Sowell, 2008). Additionally, we found that adults recruited left IFG in Broca’s area suggesting the possibility of a verbal strategy. Adults may be more efficient in maintaining items in working memory, requiring less recruitment of PFC than adolescents to perform the same computations, and may also be more likely to utilize verbal strategies to do the task.
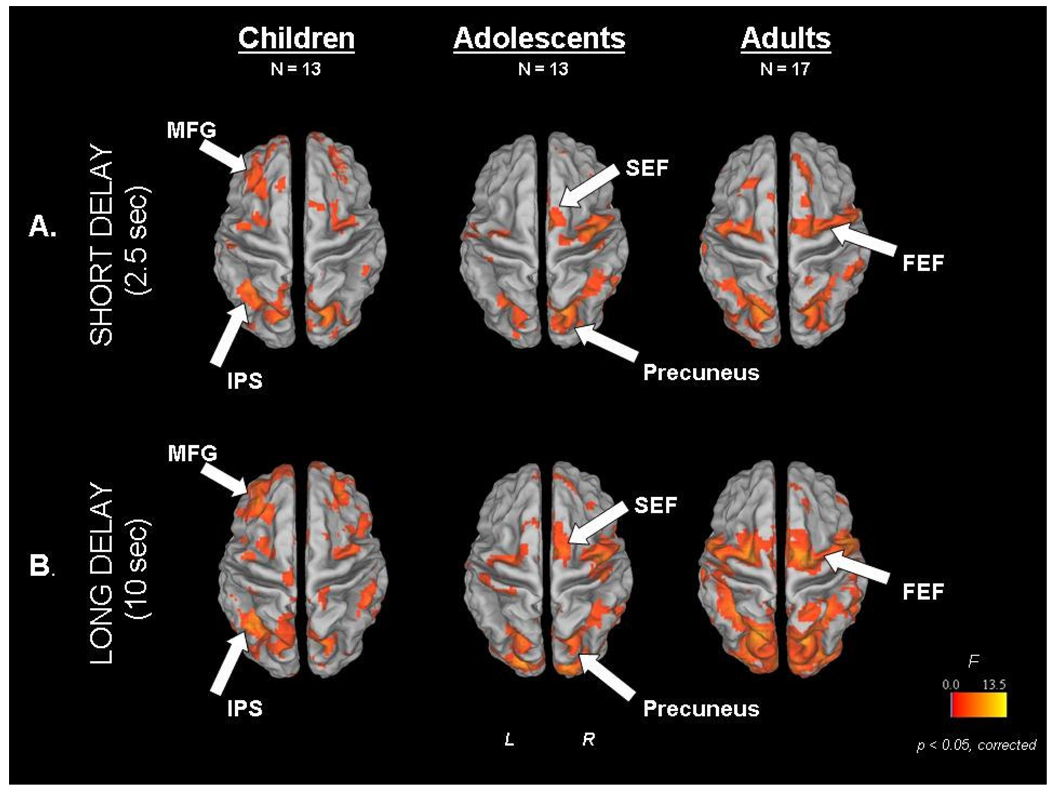
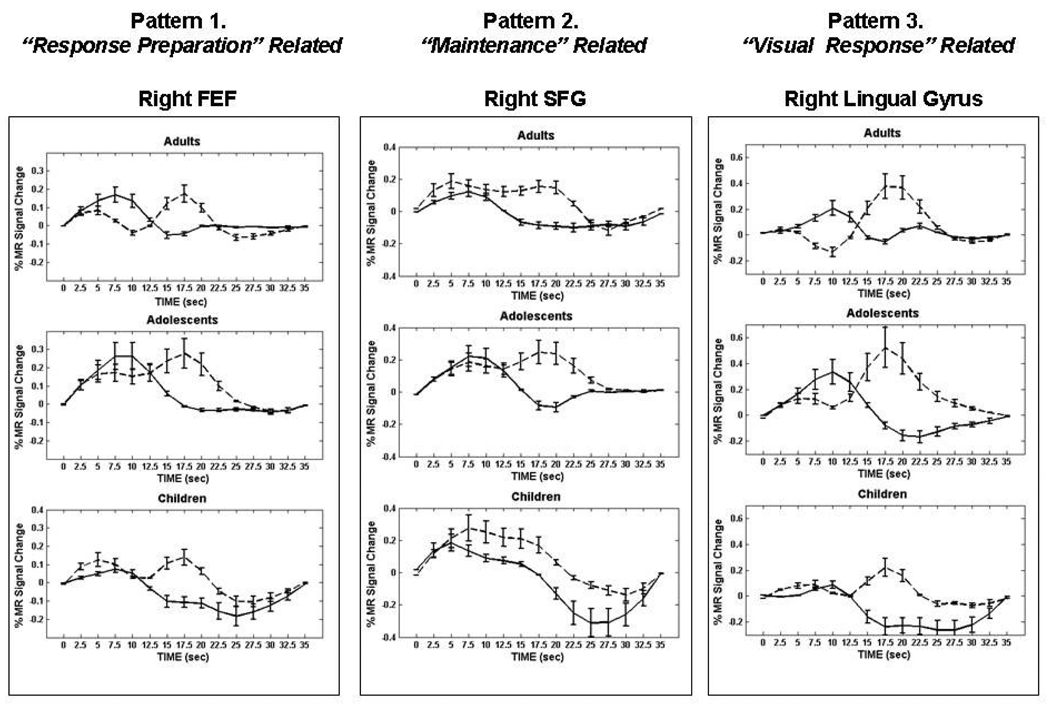
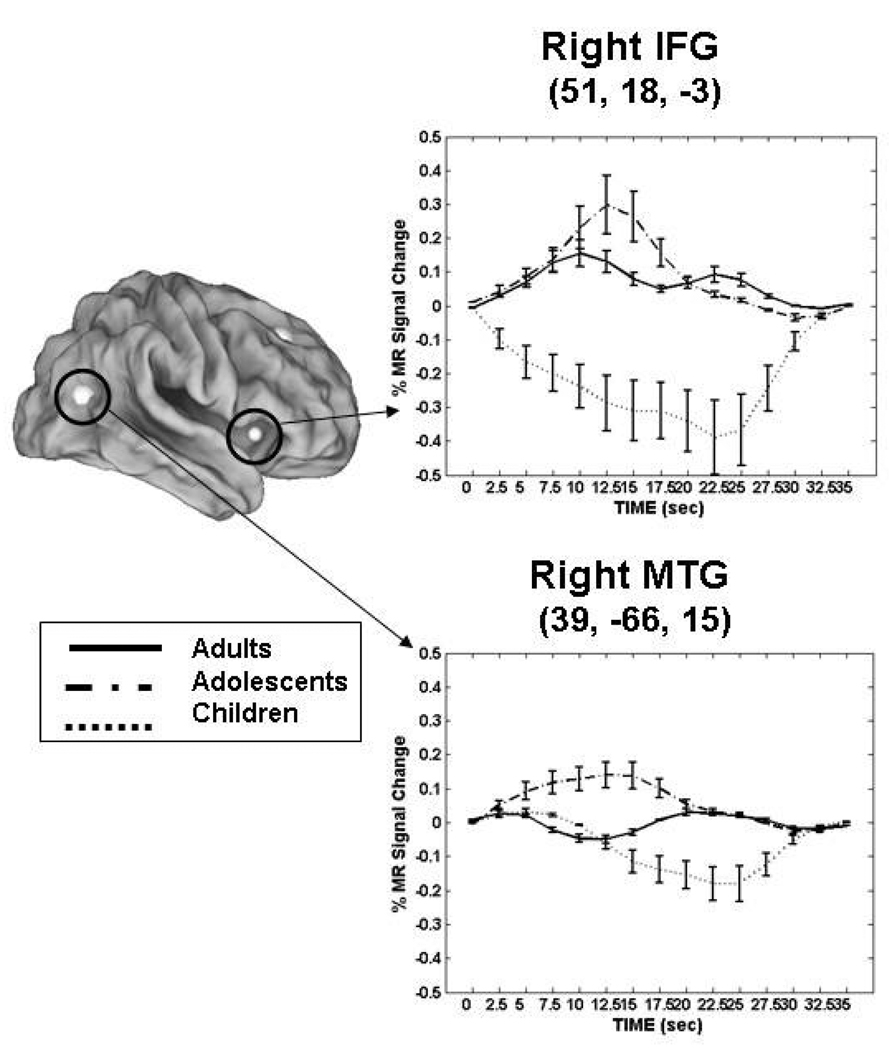
In order to better understand developmental changes related to maintenance, we performed an event-related fMRI study, which allows activity related to individual trials to be assessed, using the ODR task where the length of the delay period was varied (short 2.5sec. vs. long 10sec) to investigate developmental effects of working memory maintenance (Geier, Garver, Terwilliger, & Luna, 2009). Children (8–12 years of age), adolescents (13–17 years of age), and adults (18–30 years of age) recruited a core circuitry including frontal and parietal regions (See Figure 4) known to support working memory (Passingham & Sakai, 2004; Sweeney, Mintun, Kwee, Wiseman, Brown et al., 1995). Patterns of activity were identified for different processes involved in performing the ODR task that differed by age group, including response preparation, maintenance, and visual response (See Figure 5). During the short delays, adolescents displayed the highest magnitude of activation in the inferior frontal gyrus and middle temporal gyrus compared to children and adults (See Figure 6). During longer delay periods, children and adolescents relied on an extended circuitry including DLPFC while adults showed increased activation of parietal regions and IFG (See Figure 4). These results indicate that again, younger individuals relied more on DLPFC while adults utilized seemingly more specialized regions that support better accuracy. Areas where adults and adolescents showed magnitude differences, adolescents also showed a delay in the peak of the BOLD response similar to adults indicating approximation to adult levels (See Figure 5 Pattern 2).
Figure 4.
From (Geier, Garver, Terwilliger, & Luna, 2009). Main effect of time statistical maps for each age group and each delay trial type [A _ short delay (2.5 s), B _ long delay (10 s)], overlaid on partially inflated human PALS atlas cortical surface using Caret software. Dorsal view is shown. FEF, frontal eye field; IPS, intraparietal sulcus; MFG, middle frontal gyrus; SEF, supplementary eye field. Reprinted with permission from Journal of Neurophysiology.
Figure 5.
From (Geier, Garver, Terwilliger, & Luna, 2009). Mean BOLD time courses from representative core regions showing three observed patterns similarly across all age groups. Error bars represent SE at each time point. The y-axes for individual time course plots are scaled for best view. For each age group: long delay responses, dotted line; short delay responses, solid line. FEF, frontal eye field; SFG, superior frontal gyrus. Reprinted with permission from Journal of Neurophysiology.
Figure 6.
Adapted from (Geier, Garver, Terwilliger, & Luna, 2009). Mean BOLD time courses from regions showing age group by time interactions in short delay trials. Error bars represent SE at each time point. Adults, solid line; adolescents, dash-dot; children, dotted line. For illustrative purposes, a 4-mm sphere was centered on each peak coordinate except for the right caudate, where a 5-mm sphere was used to enable visualization on surface map. Talairach coordinates of peak voxel from each cluster are also provided. IFG, inferior frontal gyrus and MTG, middle temporal gyrus. Reprinted with permission from Journal of Neurophysiology.
Improvements in interference suppression are also evident in developmental changes in the underlying neural circuitry. A visual spatial working memory task used by (Olesen, Macoveanu, Tegner, & Klingberg, 2007) included a period of distraction in which participants had to ignore visual stimuli. Children (mean 13 years of age ± 0.5y) showed increased activity in superior frontal sulcus compared to adults (mean 23 years of age ± 3y), suggesting that they were less effective at suppressing attention to the irrelevant stimuli. Manipulation of items in working memory also results in age differences that are evident in neural activation. Adults (18–25 years of age) and adolescents (13 to 17 years of age) recruit the right DLPFC and bilateral superior parietal cortex when reversing items held in working memory (Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006), but children (8–12 years of age), who performed more poorly than older participants on this task, do not. However, children did recruit these regions during the encoding and response periods, but not when manipulation of items was required. This indicates that, while children do use similar areas to adolescents and adults, immaturities in the inhibitory system are evident in childhood and can undermine working memory performance. Overall, these studies indicate that when response inhibition and working memory are explicitly required for performance in a task, further developmental limitations in the recruitment of mature cognitive control circuitry are evident.
The implications for adolescent behavior from the working memory evidence are similar to those for inhibitory control. Although adolescents may demonstrate executive function that is equivalent to that of adults, their functional circuitry resembles that of adults performing a more difficult task. Additionally, activity becomes more distributed across brain regions with age, suggesting that in adulthood function is more evenly distributed across the brain, possibly leading to decreased reliance on prefrontal systems. This proposal is not necessarily counter to that proposed by Durston et al., (2006) of activation changing from diffuse to focal with development, a model that highlights age-related increases in activity. However, there is also evidence for age-related decreases in activity (as indicated above). Age-related increases in the engagement of a distributed circuitry does not determine the direction of activity at the regional level but emphasizes that a more extended set of regions are incorporated into the circuitry. The hypothesis, that increased functional integration across the brain is central to cognitive development, is supported by DTI studies showing that age-related improvements in working memory are related to increased structural connectivity within cortical regions and in corticosubcortical pathways (Edin, Macoveanu, Olesen, Tegner, & Klingberg, 2007; Olesen, Nagy, Westerberg, & Klingberg, 2003). These results converge with behavioral results, which provide evidence for working memory abilities in infancy but continued improvement into adolescence. Core brain regions, namely PFC, are recruited in children but refinements in the working memory circuitry continue through adolescence into adulthood, including both changes in the PFC activation and in the integration with other regions. This development reflects ongoing neural maturation, including facilitation of networks that support task-specific processing. This highlights a developmental transition towards use of a more functionally specialized, widely distributed network activity, changes which are associated with improvements in the executive function and consistent with the refinement in behavioral performance over development.
Development of Integrated Systems Level Circuitry
Studies delineating age-related changes in both response inhibition and working memory find that there are enhancements in the recruitment of widespread regions outside of PFC, including parietal, temporal, subcortical and cerebellar regions, with age (Rubia, Smith, Woolley, Nosarti, Heyman et al., 2006; Luna, Thulborn, Munoz, Merriam, Garver et al., 2001; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; O'Hare, Lu, Houston, Bookheimer, & Sowell, 2008). Indeed, PFC may be engaged only when other circuitries cannot support processing (Hazy, Frank, & O'Reilly, 2007). PFC allows the active maintenance of task information and top down modulation of action selection, and these skills may be particularly crucial in immature subjects. However, in adulthood, alternative, more posterior circuitries for learned skills may be recruited. These results indicate that developmental improvements are supported by the integration of a distributed brain system and do not solely reflect the enhanced participation of PFC.
In addition to structural white matter connectivity supporting distributed function, functional connectivity of distant regions may also support cognitive control. Functional connectivity can be assessed by cross-correlating spontaneous neural activity during rest using resting state functional MRI (rs-fcMRI). This technique provides insight into the basic connectivity of large brain circuits that may be used for cognitive processing. Fair et al (2007) assessed the strength of the connections in two circuits known to support cognitive control. The frontal-parietal network supports cognitive abilities such as inhibitory control and working memory (Dosenbach, Visscher, Palmer, Miezin, Wenger et al., 2006). The cingulo-opercular network, which includes the ACC, insula, anterior PFC, and thalamus, underlies the ability to retain a response state. Response state, mentioned previously, refers to the ability to orchestrate demands so as to apply cognitive skills in a consistent and flexible manner. Results indicated that these two circuitries continue to reorganize through adolescence, becoming more distinct and segregated from one another and further integrating long distance connections (Fair, Dosenbach, Church, Cohen, Brahmbhatt et al., 2007). Specifically, regions in medial PFC, initially part of the frontoparietal network that supports cognitive abilities such as inhibition and working memory, become part of the cingulo-opercular network supporting response state. This same group of investigators used rs-fcMRI to characterize developmental changes in the circuitry supporting the “default” system. The concept of a default system emerged from findings of a delineated circuitry which consistently decreases when we are engaged in voluntary actions requiring cognitive control (Raichle, MacLeod, Snyder, Powers, Gusnard et al., 2001). It is believed that the default system underlies the processing of internal thoughts, and is suppressed when focused attention is needed. Results indicate that, in childhood, this circuitry is weakly connected but that it becomes more strongly interconnected with development, supporting default system processing (Fair, Cohen, Dosenbach, Church, Miezin et al., 2008).
Overall, these results imply that an important part of development is the process of specializing and segregating circuitries that support task ability, response state, and default processing. Therefore, the ability to utilize cognitive control to perform a response, the ability to retain a response state, and to suppress internal thoughts improves with development, as the circuits supporting these distinct processes become independent. These results may not only be related to age-related improvements in white matter connectivity but to functional integration as seen in spontaneous waves of synchronized activity (Fair, Cohen, Dosenbach, Church, Miezin et al., 2008; Uhlhaas, Roux, Singer, Haenshel, Sireteanu et al., 2009).
Considerations in Interpreting Developmental Neuroimaging Results
While great strides have been made in understanding age-related changes in brain processes underlying cognitive development, there are still important challenges that we need to address regarding methods and the interpretation of data. A crucial feature of developmental neuroimaging studies is that adults are considered the model system. Therefore, any deviation from adult brain activity is interpreted as an immaturity. This particular aspect of interpreting developmental results can be viewed by non-developmental investigators as inconsistent, and even opportunistic, in that both increases and decreases in brain activity in adolescents compared to adults are considered a reflection of immaturity. This is an aspect of cognitive neuroimaging that is present in all studies where comparisons are made between groups, be it developmental groups or aging and patient groups. In all these cases, it is important to recognize that the nature of the group differences may not be uniform across brain systems and that results must be interpreted in the context of a model and described in a manner where clear testable hypotheses can be made. We now discuss this issue and provide suggestions for interpretation.
The dependent measure in fMRI studies -- the blood oxygen level dependent (BOLD) response -- is the microvascular response in blood flow resulting from fluctuations in the metabolic needs of a population of neurons as they become involved in a task. Developmental studies investigate age-related differences in the magnitude and time evolution of this BOLD response. However, interpreting what these differences mean in the context of development is not well-established. We know there are maturational changes in brain structure during development that impact information processing, such as synaptic pruning and myelination, as well as other changes including development of neurotransmitter processing (Geier & Luna, 2009) and hormonal influences (see other contributions to this issue). However, it is not immediately clear what the predicted change in BOLD response would be when considering these (i.e, pruning and myelination) and other (e.g., neurotransmitters, hormones) brain maturational processes. Synaptic pruning, the elimination of unused excess neuronal connections, may support more direct and less noisy computations allowing more efficient regional neural processing. This improved efficiency (decreased, but more direct neural processing) would presumably sustain more complex computations and lead to improved performance. However, how this age-related synaptic pruning would affect the BOLD response is not clear. On the one hand, fewer synaptic connections could have a lower metabolic need, resulting in a lower BOLD response in adults. This is consistent with the proposal that developmental changes follow a diffuse to focal trajectory, such as has been seen in PFC during some cognitive tasks (Casey, Trainor, Orendi, Schubert, ystrom et al., 1997; Casey, Tottenham, Liston, & Durston, 2005; Durston, Davidson, Tottenham, Galvan, Spicer et al., 2006). On the other hand, synaptic pruning may support more complex computations, and this may allow pruned regions to be recruited for a specific task that they would not support in the immature system. In this case, a region which is not involved in a task early in development may be evident in the adult system, resulting in increased activity in this region (Bunge, Wallis, Parker, Brass, Crone et al., 2005; Tamm, Menon, & Reiss, 2002).
Myelination, the thickening of the myelin sheath surrounding axons, speeds the neuronal transmission at both the regional and systems level. While myelination per se would not have a direct effect on the BOLD response, increased speed of neuronal transmission in local circuitry would enhance regional efficiency and support more complex computations locally, thus affecting the BOLD response as was suggested for synaptic pruning. Importantly, however, myelination can impact transmission at the systems level, allowing for the integration of widely distributed circuitries supporting top-down modulation of behavior. This top-down modulation may be essential for executive function (Goldman-Rakic, Chafee, & Friedman, 1993b). A more distributed network in the adult could result in a lower BOLD response of executive regions such as PFC, while other regions, more specialized for the task at hand, are recruited. A related concern is that age-related changes in brain structure can undermine the ability to assess true developmental change. However the gross morphology of the brain is in place by mid-childhood (Goldman-Rakic, Chafee, & Friedman, 1993a; Caviness, Jr., Kennedy, Richelme, Rademacher, & Filipek, 1996; Giedd, Snell, Lange, Rajapakse, Casey et al., 1996) so that size and organization of brain anatomy are roughly equivalent in adolescents and adults (Schlaggar, Brown, Lugar, Visscher, Miezin et al., 2002b). There are also concerns that age-related vascular changes may undermine the ability to discern true differences in the BOLD response. However, studies comparing the bold response across the brain at different ages indicate that this is not a problem (Kang, Burgund, Lugar, Petersen, & Schlagger, 2003). While gross structural changes of the brain are complete early in development, there are continued refinements in brain structure that are precisely the changes that many studies seek to identify.
It is not generally clear if changes in the BOLD response reflect age-related changes in brain processing, or differences in the use of strategies which recruit a distinct circuitry. For instance, adults may use verbal strategies to enhance cognitive performance. Therefore, it is possible that BOLD increases and decreases associated with performance are not related to age-related changes in brain processes per se, which may be mature, but instead to age-related differences in psychological processes. This is particularly a concern when performance differs by age. Differences in performance could reflect that the younger group uses a different strategy, with a distinct circuitry, from adults or that they utilize a comparable strategy as adults but use a suboptimal (immature) version of the mature circuitry. The former does not show developmental differences in brain function per se, but instead in psychological function, while the latter probably does reveal true developmental differences in brain function. However, while characterizing both changes is crucial for understanding development, distinguishing between these possibilities is not straightforward. While changes in behavior have long led to hypotheses about differences in brain function, it is now evident that changes in the pattern of brain function can provide insight into what psychological changes co-occur with development. In other words, the network of areas used in adolescence and how closely related it is to that used in adulthood provides insight to what psychological processes are changing.
Several approaches have been used to control for the effects of strategy use, which can confound the ability to characterize developmental changes. One approach is to equate performance across ages. This can provide important information about specific differences in brain circuitry that support similar performance at different ages (Schlaggar, Brown, Lugar, Visscher, Miezin et al., 2002b). Developmental differences in brain function supporting equivalent performance can reflect that greater effort is being exerted by the immature group, compensatory brain processes are being used due to limitations in accessing the correct circuitry, or that distinct strategies are being used. Characterizing differences in effort is important because it suggests that the basic circuitry is available but has not yet reached the mature processing level evident in the adult system. That is, the immature system may use more neural tissue or recruit this system for a longer period, to process the same neural computations as the mature system. This approach can also show alternate circuitries that are used as a compensatory mechanism as well as elucidate what parts of the circuitry are not being accessed. For example, young subjects’ IFG activation, associated with inhibitory processing, may not be able to support quick inhibitory responses. Instead parietal cortex, which supports control of attention and visuospatial processing, may be used by adolescents (Schlaggar, Brown, Lugar, Visscher, Miezin et al., 2002a). However, care must be taken that a bias in sampling does not occur when performance is equated -- that is, that exceptional children are not being compared to adults with unusually poor performance.
Alternatively, evidence of age-related differences in performance can also elucidate how each age is performing the task, including the use of different strategies. If adolescents do not use the brain systems evident in adults, this may reflect limitations in accessing the mature circuitry, which leads to using a different compensatory circuitry or strategy. One way to reconcile such a difference (strategy use vs. true inability to access a given circuitry) is to use a parametric approach where cognitive load is manipulated, and the regions sensitive to these manipulations can be examined. On the one hand, younger subjects could show evidence of recruiting a given circuitry during low cognitive load but then use a different circuitry when load increases, indicating that the circuitry is in place but not mature enough to support tasks with high cognitive loads. On the other hand, younger subjects could show an inability to access a given circuitry regardless of cognitive load or performance differences, suggesting that they are using an alternative approach regardless of task difficulty, possibly due to limitations in accessing the optimal strategies and circuitry (Rubia, Smith, Taylor, & Brammer, 2007).
Another approach to control for performance is to investigate differences in activation at the trial level. Even if there are age-related differences in performance across a task, examining correct and error trials separately can be used to equate performance and elucidate differences in brain function (Velanova, Wheeler, & Luna, 2008). It is still possible that different strategies are being used to achieve similar overall performance. However, at the trial level, the same response is being generated and developmental differences in the circuitry supporting the same behavior can elucidate true age-related differences in brain processing. Training to a criterion can also be used to control for performance differences and, if a strategy is provided, it can also control for strategy use (Rubia, Smith, Taylor, & Brammer, 2007). However, this technique may decrease the sensitivity to discriminate developmental differences important in every-day life. Differences in strategy use can also be seen as akin to learning. Neuroimaging studies of learning in adults indicate that the circuitry that is initially recruited when a task is novel is qualitatively different from the one that is recruited once the behavior has been learned (Ungerleider, Doyon, & Karni, 2002). Similarly, cognitive control in the adolescent could approximate the “pre-learned” state evident in the adult. That is, the adult has more ease in exerting cognitive control than the adolescent, since some aspects of cognitive control have been “learned” and can be processed more automatically.
Direction of Developmental Differences
When age groups differ in activation, qualitatively different processes may be involved, each with distinct interpretations. One common result is that the younger group demonstrates an increased magnitude of activity compared to adults in an equivalent region. When the same circuitry as adults is being accessed by younger subjects, but the younger subjects show higher activity, this is often interpreted as indicating that greater effort is required for younger subjects to do the task (Tamm, Menon, & Reiss, 2002; Luna, Garver, Urban, Lazar, & Sweeney, 2004). This interpretation is based on adult studies showing that activation increases with cognitive load (Keller, Carpenter, & Just, 2001). On the other hand, results indicating that younger subjects show decreased activity in an equivalent region along with poor performance compared to adults, suggests that younger subjects are unable to access the mature and presumably optimal circuitry. Both results could indicate that the brain circuitry is available but immature, with the neural mechanisms not processing as efficiently as they do in the mature system. Increased efficiency, on the one hand, can refer to adults being able to perform the same computations as younger subjects with less neural processing. On the other hand, increased efficiency could be interpreted as also underlying increased activity in adulthood, compared to younger subjects, reflecting that mature neural processing is able to support computations that the immature system can not yet support. That is, the adult may recruit neural systems that may not be accessed by younger subjects because their local circuitry is immature (for example, less-pruned) or connectivity is immature and cannot quickly access a region to support complex behavior. The former possibility implies that the basic circuitry is available to children but still immature and inefficient, hence children need to drive it to higher magnitudes, which is possibly related to effort. The latter result suggests that there are actual limitations prior to adulthood in the computational ability of the system to support higher levels of activity, undermining its use. Additionally, how to interpret increases and/or decreases in activation draws attention to the importance of the baseline task. Developmental differences in the baseline comparison task can undermine the ability to assess developmental changes in the experimental task (Marsh, Zhu, Schultz, Quackenbush, Royal et al., 2006b).
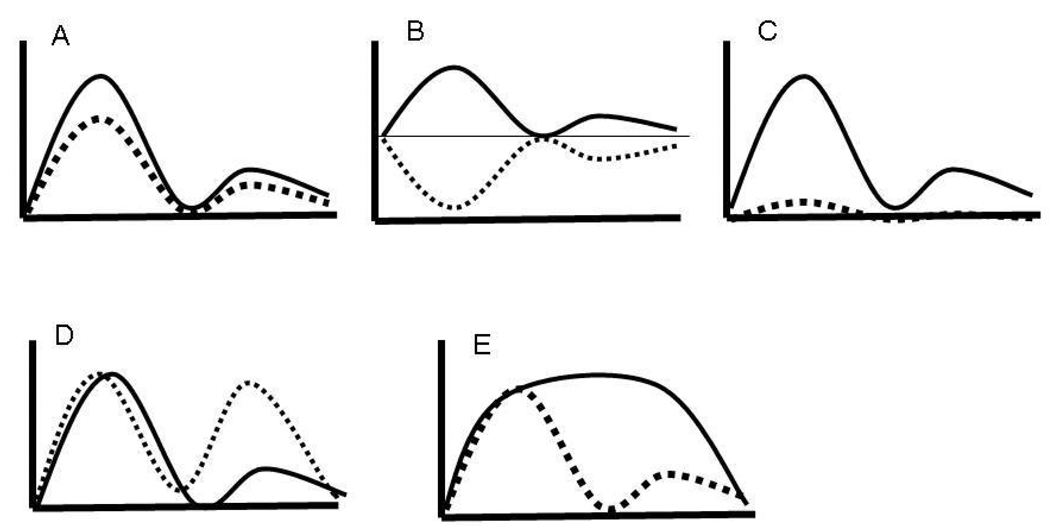
Many of these concerns can be addressed, and insight into the meaning of developmental differences can be gained, by comparing the BOLD time courses generated from the regions of interest across age groups. Age-related differences in magnitude could be due to distinct differences in the BOLD response that would appear equivalent in an activation map but are qualitatively different, such as when both groups have a comparable timecourse but one has a higher magnitude of activity (Figure 7a) or deactivation of the same region by one group (Figure 7b) or a failure of one group to recruit the region (Figure 7c), or one group fails to exhibit a double peak (Figure 7d); or display a sustained response (Figure 7e). These different patterns of group effects could have distinct implications, for instance either a simple difference in effort (Figure 7a) or a difference in the specific task component that is supported by that region, such as maintenance or response preparation (Figure 7d and e). In this manner, “increases” in activity can be quantified in relation to whether there is a decrease, lack of recruitment, or difference in the shape of the time courses between groups (Marsh, Zhu, Schultz, Quackenbush, Royal et al., 2006a). Several statistical approaches can be used to test for age-related differences, including repeated measures analyses to test for magnitude differences (for each TR of the experiment), the magnitude of peak activity, the time of peak (younger subjects may have a delay in processing information in a region delaying the peak of the BOLD response), or the response shape (double peaks, or prolonged peaks reflecting sustained processes (Geier, Garver, & Luna, 2007a; Geier, Garver, & Luna, 2007b). Double peaks are still not well understood and are usually not considered. However, if these appear consistently across subjects within an age group and if there are typical time courses evident in other regions of the brain, the meaning of this response type should be addressed.
Figure 7.
Idealized depiction of developmental changes in BOLD timecourses: a) similar time courses but one group shows lower magnitude; b) deactivation of the same region by one group; c) Only one group recruits a region; d) one group fails to exhibit a double peak; and e) one group fails to show a sustained response.
Another methodological issue that affects how we interpret neuroimaging results is block vs. event-related designs. fMRI studies can be performed using a blocked design, where brain activity represents the collective activity of a block of trials, or an event-related design where brain activity is assessed at the single-trial level. The blocked design offers an optimal signal to identify the brain regions participating in a task and may help characterize a ‘response state’. However, correct and incorrect trials are grouped together. Event-related designs allow the characterization of brain function that underlies trial types (e.g., correct vs. incorrect; different cognitive loads). While this necessitates more trials, it allows for only correct trials (or incorrect, if there are enough) to be assessed, assuring that the comparisons between ages are more appropriate since the same behavior is being examined. A particularly fruitful approach is the mixed block event related fMRI design that permits the assessment of correct and incorrect trials as well as the block level processes that can reflect the status of response state (Velanova, Wheeler, & Luna, 2008). As mentioned previously, differences in processes supporting the ability to retain a response state may be crucial to understanding developmental improvements in cognitive control during adolescence.
It is also important to balance the benefits of a theory-driven ROI analysis with the need for more exploratory analyses of development to identify distinct regions or increased variability in children compared to adults. Limiting studies to only investigating regions already implicated in the literature, such as PFC, undermines the ability to characterize system level changes that may affect integration of the PFC with other regions. Studies usually provide both approaches, allowing confirmation with the previous literature as well as extension to new areas. Finally, the ages chosen to represent different developmental stages can influence outcomes. Some studies group children and adolescents into a homogenous group (Rubia, Overmeyer, Taylor, Brammer, Williams et al., 2000; Rubia, Smith, Woolley, Nosarti, Heyman et al., 2006), limiting the ability to see stage-like differences in development that may be especially pertinent to the transition through adolescence (e.g., see below for “U” shaped function of executive regions in adolescence) and developmental differences in general. Following a large sample in a longitudinal fashion provides the most powerful and sensitive approach to characterizing different profiles of development, including stage-like phases and the nature of individual differences. However, there are drawbacks to these types of designs, since they are expensive and time-consuming, and take longer to produce results.
Conclusions
The developmental fMRI literature characterizing age-related differences in cognitive control includes a range of methodological approaches. This work has generated diverse findings that still share some important consistencies. As has been proposed in the literature, prefrontal systems play a primary role in executive processes and have a protracted development (much like other association areas) into adolescence. Studies therefore have used PFC as an a priori region of interest and have shown apparent inconsistencies. Results indicate both age-related increases (Rubia, Overmeyer, Taylor, Brammer, Williams et al., 2000; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Tamm, Menon, & Reiss, 2002) and decreases (Rubia, Smith, Taylor, & Brammer, 2007) in prefrontal participation. Studies where performance is equated have found that DLPFC activation, a region which supports working memory, response planning, and regulation needed for cognitive control, decreases with age (Tamm, Menon, & Reiss, 2002). This increased activation in children and adolescents suggests that adult-level performance on executive tasks in these younger participants may require increased effortful attention, therefore necessitating more pronounced reliance on DLPFC. These results could also reflect immaturities in the structure of PFC, which could result in a more prolonged or extended computational process, generating increased activity compared to the adult system. Results also provide evidence for age-related increases in PFC activation, as well as in regions connected to PFC, including striatal and parietal regions. These increases could also reflect changes in brain structure that, as adolescents reach adulthood, allow PFC regions to sustain complex computations locally and integrate function across the brain leading to increased recruitment of these regions to support voluntary control. There are also studies that find an inverted U-shaped function in activation where adolescents may show increased activity of executive regions in comparison to both children and adults (Ciesielski, Lesnik, Savoy, Grant, & Ahlfors, 2006; Luna, Thulborn, Munoz, Merriam, Garver et al., 2001). This result is particularly significant, because it shows that adolescence is a unique stage of development -- while performance is approximating that of adults, and important aspects of the circuitry are in place, there are still immaturities in adolescence that limit the flexible use of cognitive control, supported by the PFC. Importantly, results show that there are limitations during adolescence in specific components of cognitive control such as error processing, processing complex tasks, and retaining a control state (Velanova, Wheeler, & Luna, 2008; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006).
What is evident across studies is that prefrontal systems and the ability to recruit distributed function are present early in development. However, recent work indicates that the connections within these distributed circuitries increase in strength, and incorporate more long range connections, through adolescence. The transition from adolescence to adulthood therefore can be seen as a change in mode of operation from initially relying on more regionalized processing, such as in the PFC, earlier in development to relying on a broader network of regions that share processing in an efficient and flexible manner at the systems level. Children may rely on processes that support aspects of executive control more generally, while adolescents may transition to utilizing multiple, posterior regions specialized for specific aspects of a task that together provide a rapid response tailored to the task, freeing up executive regions for more complex duties. This transition in the mode of operation may be supported by structural brain changes or other brain dynamics such as brain synchrony that encourage recruitment of more wide-range networks. In adulthood, brain systems may be better specialized and may more efficiently interact with other distant regions, providing an circuitry that supports flexible cognitive control. While brain function in individuals with psychopathology may be atypical throughout development, their impairments may become evident during adolescence when the transition to relying on different brain circuits, which may have always been abnormal, occurs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop Color-Word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Amso D, Johnson SP. Selection and inhibition in infancy: evidence from the spatial negative priming paradigm. Cognition. 2005;95:B27–B36. doi: 10.1016/j.cognition.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford University Press: New York; 1986. [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Developmental Neuropsychology. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Bjorklund DF, Harnishfeger KK. The resources construct in cognitive development:diverse sources of evidence and a theory of inefficient inhibition. Developmental Review. 1990;10:48–71. [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Development of frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. The Journal of Neuroscience. 2005;25:10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, ystrom LE, iedd JN, astellanos FX, axby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: A volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6:736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Petenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP. Developmental neural networks in children performing a Categorical N-Back Task. NeuroImage. 2006;33:980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Cowan N, Saults JS, Morey CC. Development of Working Memory for Verbal-Spatial Associations. J Mem Lang. 2006;55:274–289. doi: 10.1016/j.jml.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. Journal of Neuroscience. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca CR, Wood SJ, Anderson V, Bucanan J, Proffitt TM, Mahony K. Normative data from the Cantab: I. Development of executive function over the lifespan. Journal of Clinical & Experimental Neuropsychology. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: efficiency, working memory, and thinking. Monogr Soc Res Child Dev. 2002a;67:1–155. discussion 156. [PubMed] [Google Scholar]
- Demetriou A, Christou C, Spanoudis G, Platsidou M. The development of mental processing: Efficiency, working memory, and thinking. Monographs of the Society for Research in Child Development. 2002b;67:1–155. discussion 156. [PubMed] [Google Scholar]
- Dempster FN. Memory span: Sources of individual and developmental differences. Psychological Bulletin. 1981;89:63–100. [Google Scholar]
- Diamond A, Goldman-Rakic PS. Comparison of human infants and rhesus monkeys on Piaget's AB task: evidence for dependence on dorsolateral prefrontal cortex. Experimental Brain Research. 1989;74:24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Diamond A, Towle C, Boyer K. Young children's performance on a task sensitive to the memory functions of the medial temporal lobe in adults: The delayed nonmatching-to-sample task reveals problems that are due to non-memory-related task demands. Behavioral Neuroscience. 1994;108:659–580. doi: 10.1037//0735-7044.108.4.659. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen PJ, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. Journal of Cognitive Neuroscience. 2007;19:750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Hatta T, Fukushima K. Development of voluntary control of saccadic eye movements. I. Age-related changes in normal children. Brain & Development. 2000;22:173–180. doi: 10.1016/s0387-7604(00)00101-7. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behavioural Brain Research. 1997;84:203–223. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends in Neurosciences. 1997a;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 3 ed. Raven Press: New York; 1997b. [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. Journal of Neurophysiology. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuoimage. 2007b;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. Neuoimage. 2007a;35:904–915. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. The Maturation of Incentive Processing and Cognitive Control. Pharmacology Biochemistry and Behavior. doi: 10.1016/j.pbb.2009.01.021. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Chafee M, Friedman H. Allocation of function in distributed circuits. In: Ono T, Squire LR, Raichle ME, Perrett DI, Fukuda M, editors. Brain Mechanisms of Perception and Memory: From Neuron to Behavior. Oxford University Press: New York; 1993b. pp. 445–456. [Google Scholar]
- Goldman-Rakic PS, Chafee M, Friedman H. Allocation of function in distributed circuits. In: Ono T, Squire LR, Raichle ME, Perrett DI, Fukuda M, editors. Brain Mechanisms of Perception and Memory: From Neuron to Behavior. Oxford University Press: New York; 1993a. pp. 445–456. [Google Scholar]
- Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T.O.V.A.) Journal of Child Psychology and Psychiatry and Allied Disciplines. 1993;34:1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus:computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. Journal of Neurophysiology. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlagger BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension:a fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kok A. Varieties of inhibition: manifestations in cognition, event-related potentials and aging. Acta Psychologica. 1999;101:129–158. doi: 10.1016/s0001-6918(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, Mattson AJ. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Logan GD, Gordon SE. Executive control of visual attention in dual-task situations. Psychological Review. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developental Neuropsychology. 2002;22:595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: A strategy for testing neurodevelopmental hypotheses. Schizophrenia Bulletin. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. Cognitive development: fMRI studies. In: Keshavan MS, Kennedy JL, Murray RM, editors. Neurodevelopment and Schizophrenia. Cambridge University Press: London/New York; 2004a. pp. 45–68. [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004b;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006a;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006b;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews in Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwitt CL. Functional neuroanatomy of spatial working memory in children. Developmental Psychology. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. NeuroImage. 2008;42:1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain Activity Related to Working Memory and Distraction in Children and Adults. Cerebral Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cognitive Brain Research. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Current Opinion in Neurobiology. 2004;14:163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Petit L, Courtney SM, Ungerleider LG, Haxby J. Sustained activity in the medial wall during working memory delays. Journal of Neuroscience. 1998;18:9429–9437. doi: 10.1523/JNEUROSCI.18-22-09429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, Taich AM, D'Esposito M. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. Journal of Cognitive Neuroscience. 2000;12:2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. Journal of Cognitive Neuroscience. 2006;18:1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior:effects of age and irrelevant information on the ability to inhibit one's actions. Acta Psychologica. 1999;101:315–337. [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GP, Bashore TR. Sources of interference from irrelevant information: a developmental study. Journal of Experimental Child Psychology. 1997;65:315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Current Opinion in Neurobiology. 2007;17:228–233. doi: 10.1016/j.conb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18:1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002b;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. 2002a. Science;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Reserach. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Berman RA, McCurtain BJ, Strojwas MH, Voyvodic JT, Genovese CR, Thulborn KR. fMRI studies of spatial working memory. Society For Neuroscience. 1996;22(6646):1688. Ref Type: Abstract. [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. PET studies of voluntary saccadic eye movements and spatial working memory. Schizophrenia Research. 1995;15:100. doi: 10.1152/jn.1996.75.1.454. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Takarae Y, Macmillan C, Luna B, Minshew NJ. Eye movements in neurodevelopmental disorders. Current Opinion in Neurology. 2004;17:37–42. doi: 10.1097/00019052-200402000-00007. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. Journal of Experimental Child Psychology. 1989;48:353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenshel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiology of learning and memory. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Van den Wildenberg WPM, van der Molen MW. Developmental trends in simple and selective inhibition of compatible and incompatible responses. Journal of Experimental Child Psychology. 2004;87:201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, van der Molen MW. Developmental trends for object and spatial working memory: a psychophysiological analysis. Child Development. 2007;78:987–1000. doi: 10.1111/j.1467-8624.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational Changes in Anterior Cingulate and Frontoparietal Recruitment Support the Development of Error Processing and Inhibitory Control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wise LA, Sutton JA, Gibbons PD. Decrement in Stroop interference time with age. Perceptual and Motor Skills. 1975;41:149–150. [Google Scholar]