Abstract
Opioid receptor like-1 receptor (ORL1) is selective for nociceptin/OFQ, a peptide linked to stress. Since immunologic stimuli exert stressor-like effects, the neuroendocrine and behavioral effects of the T-cell superantigen staphylococcal enterotoxin A (SEA) were tested in ORL1−/− and ORL1+/+ wildtype 129/S6 mice. Within 2hrs of SEA challenge both genotypes showed elevated corticosterone, but only wildtypes were elevated after 4 hrs, and had altered hypothalamic CRH mRNA. Although amygdaloid CRH and TNFα mRNA was increased by SEA, this did not vary with genotype. Interestingly, gustatory neophobia due to SEA challenge was augmented in ORL1−/− mice, although object neophobia tested 4 days later was abrogated. These results suggest differential requirements for ORL1 in the mediation of neuroimmune effects exerted at different times after an immune challenge.
Keywords: Corticosterone, stress, anxiety, staphylococcal enterotoxin A, ORL1 receptor, cytokines
1. Introduction
OrphaninFQ/Nociceptin (OFQ/N) is a heptadecapeptide similar in homology to the classical opioid peptide dynorphin A (Mogil and Pasternak, 2001), and was found to be the endogenous agonist of the opioid receptor-like 1 (ORL1) receptor (Meunier et al., 1995; Reinscheid et al., 1995). Similarly, the ORL1 receptor shares high homology with traditional opioid-like receptors (Meunier et al., 1995), yet neither OFQ/N nor ORL1 interact with components of the classical opioid system (Bunzow et al., 1994; Mollereau et al., 1999; Mollereau et al., 1994; Reinscheid et al., 1996). Although OFQ/N is largely implicated in nociception (Zeilhofer and Calo, 2003), its modulatory role on anxiogenic like behavior is of great interest. For example, several studies have reported an anxiolytic role for OFQ/N (Ciccocioppo et al., 2004; Gavioli et al., 2007; Griebel et al., 1999; Jenck et al., 1997; Jenck et al., 2000) suggesting that activation of this system may be necessary to negatively regulate anxiogenic processes and to control behavioral responses to stress that are associated with anxiety states. Alternatively, an anxiogenic role for OFQ/N has been proposed (Devine et al., 2001; Fernandez et al., 2004).
Previously, in mice, intracerebroventricular (icv) administration of OFQ/N increased exploration of the lit compartment of a standard dark-light box (Jenck et al., 1997). In rats, OFQ/N treatment increased the number of entries and time spent in the open arms of an elevated plus maze (Jenck et al., 1997). Conversely, Koster, Montkowski et al (1999) determined that OFQ/N-deficient (OFQ/N−/−) mice displayed increased levels of anxiety-like behavior when tested in an open field apparatus. Specifically, OFQ/N−/− mice spent less time in the center-most region of the open field and displayed reduced levels of locomotor activity when compared to wildtype mice (Koster et al., 1999).
Stress as a physiological state originates from both systemic and psychogenic origins impacting central nervous system (CNS) functioning and activating the hypothalamic-pituitary-adrenal (HPA) axis. A number of immunologic stimuli, such as lipopolysaccharide (LPS) and proinflammatory cytokines induced by LPS, such as IL-1β, TNFα, and IL-6 have exerted significant activational effects on the HPA axis and various sickness behaviors, such as anorexia, reduced locomotion, altered sleep and fever (Dantzer, 2009). Therefore, immunologic stimuli represent one category of stressors that can alter neuroendocrine and behavioral functioning. While many immunologic stimuli shown to have stressor-like effects have engaged the innate arm of the immune system, bacterial T cell superantigens (e.g., staphylococcal enterotoxin A and B; SEA and SEB) have similarly produced HPA axis activation and other stress-related effects (Kaneta and Kusnecov, 2005; Kusnecov et al., 1999), including increased expression of CRH mRNA (Kusnecov et al., 1999) and c-Fos-immunoreactive cells (Rossi-George et al., 2005) in hypothalamic and amygdaloid regions, areas known to modulate emotionality. Furthermore, SEA challenge was shown to increase mRNA levels for the precursor molecule of OFQ/N (ppOFQ/N) in amygdaloid and hypothalamic regions of C57BL/6J mice (Kawashima et al., 2002).
Thus, given the hypothesized role of the OFQ/N-ORL1 system in modulation of the behavioral response to stress (Ciccocioppo et al., 2004; Jenck et al., 1997; Koster et al., 1999), and that mRNA levels for ppOFQ/N are increased in response to immunological challenge (Kawashima et al., 2002), activation of the ORL1 receptor may be necessary to negatively regulate HPA activity and anxiogenic like behaviors observed subsequent to systemic immune stressors. Therefore, the present study sought to determine the immunological, endocrine and behavioral effects of acute exposure to SEA in male 129S6 ORL1 receptor wildtype (ORL1+/+) and knockout (ORL1−/−) mice. Specifically, it was hypothesized that ORL1−/− mice would show augmented HPA activity, immunological responding, anxiety-like behavior and anorexia following acute SEA injection relative to ORL1+/+ mice.
2. Materials and Methods
Animals
Male 129S6, ORL1+/+ and ORL1−/− mice, were initially bred in the vivarium of Robert Wood Johnson Medical School, University of Medicine and Dentistry, New Jersey. After weaning, animals were housed 3–4 per cage under a 12:12 h light:dark cycle (lights on 0600h) in the Psychology Department, Rutgers University. Food and water were available ad libitum and animals were allowed two weeks acclimation prior to the start of testing. Animals were 5–6 months of age at the time of experimentation. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health, and approved by the Rutgers Institutional Animal Care and Guidance Committee.
Experimental Procedures
In all experiments animals were injected intraperitoneally (IP) with either staphylococcal enterotoxin A (5μg/animal) or physiological saline in a volume of 0.2 mL. All injections were given between 0900 and 1000 h. For studies involving biological measures, animals were sacrificed 2 or 4 h after a single injection. In one experiment, two sessions of behavioral testing occurred after an initial injection with SEA or Saline. This involved food intake measures at 2 and 4 h after injection, and exposure to an open field and novel object on Day 4 (see below for behavioral methodology).
Biochemical Assays
Corticosterone measures
After injection of SEA or saline, animals were sacrificed by rapid decapitation and trunk blood was collected into EDTA-treated vacutainer tubes. Blood was then centrifuged for 20 minutes at 2500 RPM, and plasma was collected and stored at −70°C until ready for corticosterone assay using a commercially available, radioimmunoassay kit (MP Biomedicals, Solon, OH, USA).
Splenic Protein and Cytokine Quantification
Spleens were dissected and placed on dry ice until storage at −70°C. For protein extraction, spleens were homogenized in1 ml of 1mM phenylmethanesulfonyl fluoride (PMSF) in 0.1 M phosphate buffer to inhibit protease activity and centrifuged for 30 min at 3500 RPM. The supernatant was collected and measured for protein and cytokine concentration. Total protein was quantified using the BCA protein assay kit (Pierce, Rockford, IL, USA). Absorbance was read at 562 nm (using EL800 universal BioTek microplate reader) with concentrations (μg/ml) calculated from a standard curve generated using bovine serum albumin (BSA).
Following protein quantification, tumor necrosis factor alpha (TNFα) and interleukin-2 (IL-2) was determined using ELISA kits purchased from eBiosciences. The supernatants from the spleen homogenates (see above) were assayed at a 1:4 dilution for detection of TNFα and at a 1:40 dilution for IL-2. All cytokine standards and samples were run in duplicate and concentrations calculated from a standard curve using KC Junior software (BioTek) and expressed as pg/ml. Subsequently, cytokine concentrations were adjusted per μg of protein.
Brain dissection and RNA extraction
Upon sacrifice of animals via rapid decapitation, brains were removed and immediately frozen in 2-methylbutane, placed on dry ice and stored at −70°C until ready for dissection of the hypothalamus and amygdala, as previously described (Kawashima et al., 2002). These brain regions were subjected to total RNA extraction using TRIzol® reagent as per manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Briefly, tissue was homogenized in Trizol solution, incubated (for five minutes) at room temperature and then chloroform extracted (.2mL chloroform/1mL Trizol). This was followed by centrifugation at 3500 RPM for 15 min, collection of supernatant and addition of isopropanol for RNA precipitation (.5mL isopropanol/1mL Trizol). The RNA was pelleted by centrifugation, washed in 75% ethanol and finally dissolved in RNAse-free DEPC treated water. The concentration of RNA was quantified (ng/ul) using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and stored at −70°C until ready to be assayed.
Reverse Transcription and RT-PCR
Following RNA extraction 1 ug of RNA was reversed transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA). cDNA was then assayed by real-time PCR (Applied Biosystems 7900HT system) for quantification of mRNA expression for corticotropin releasing hormone (CRH), TNFα, and TNF receptor 1 (TNFR1) (Kohman R.A., in press; Rossi-George et al., 2005). Sample values were calculated from standard curves generated using a twofold serial dilution of undiluted control cDNA obtained from splenic, hypothalamic or amygdaloid tissue of an animal that was given SEA. This method allowed for relative quantification of mRNA expression within each sample for each gene of interest. Thus, for all samples mRNA was expressed as a ratio of the gene of interest to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in arbitrary units based on the standard curve.
Behavioral Testing
Food Consumption
Animals were injected with SEA or saline and tested for consumption of a novel liquid diet (Prosobee baby formula) in a novel context. This testing situation has previously been shown to elevate plasma ACTH, likely due to neophobia (Kusnecov et al., 1999). No food or water deprivation was necessary prior to testing, since untreated and non-deprived mice can drink up to 1 ml of the Prosobee liquid over a 1 hr period. Therefore, given that water deprivation may produce stressor-like effects, no deprivation schedule was instituted. For testing, animals were removed from their home cages and placed into individual, opaque, shoe box cages. A tube of Prosobee solution was then placed through the cage top and made easily accessible to the animal. Consumption was measured across two consecutive tests. Each test lasted 1 hr with the first commencing 2 hr after injection and the second test commencing 4 hr after injection. Animals remained in the novel cages between consumption periods. At the end of each consumption test, tubes were weighed and subtracted from pre-test weights. At the end of the second consumption test, animals were returned to their home cages until Day 4 after SEA or saline injection when they were subjected to non-appetitive, exploratory testing in the open field as described in the next section.
Open field and novel object testing
The open field/novel object test was conducted as previously described (Cooper and Kusnecov, 2007). Briefly, exploratory behavior was measured in the absence, and then presence of a novel object. The apparatus measured 63 × 57 × 28 cm (LxWxH) and was made of opaque Plexiglas. The area was enclosed by a white curtain, and an overhead video camera connected to a VHS tape player recorded all movements. For the test, animals were allowed to freely explore the field for 5 minutes, at the end of which a novel object (metal cylinder measuring 6 cm diameter × 15 cm height) was placed into the center of the field for the remaining 5 minutes of the test. Therefore, total exposure time to the open field was 10 minutes. At the end of the test, animals were returned to their home cage.
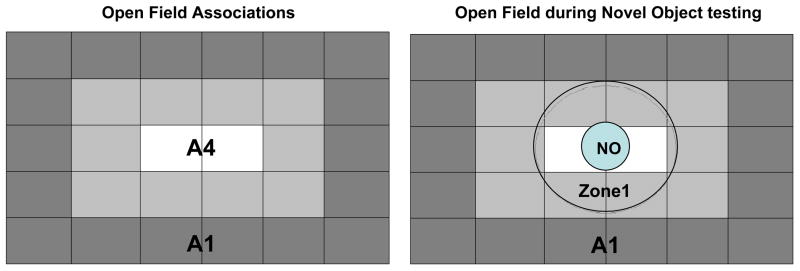
Exploration was measured on the recorded videotapes using video-tracking software (Spontaneous Motor Activity Recording and Tracking (SMART), San Diego Instruments, San Diego, CA, USA). For purposes of analysis, the software was used to demarcate areas of the field into peripheral and central areas (See Figure 1). Specific behaviors measured during both phases of testing (open field and novel object) included total distance traveled in the various regions (with and without the novel object), percentage of time spent within the innermost area prior to (association area 4; A4) and during (circumscribed by zone 1) the presence of the novel object itself (see Figure 1). In the presence of the novel object, latency to enter Zone 1 for the first time was measured, followed by the number of entries into the region surrounding the novel object. Additional videotaped observations included number of contacts and rearings made with the novel object. Number of contacts and rearings with the novel object were summed and analyzed as total number of interactions.
Figure 1.
Schematic of open field/novel object zones. During open field testing (prior to novel object [NO] presentation) A4 was designated as the center, while A1 was designated the periphery. Upon introduction of the NO, the area surrounding the object, zone 1, became the designated central area, while A1 remained the periphery.
Data Analysis
Data were analyzed by ANOVA, and where appropriate using repeated measures. Fisher’s LSD was used to determine significant differences in the case of a main effect. Significance was set at p < 0.05.
3. Results
1. Immunological and Neuroendocrine Effects of Acute SEA Exposure in 129S6 ORL1+/+ Receptor Wildtype and ORL1−/− Receptor Knockout Mice 2 and 4 hours Following Acute SEA or Saline Injection
1.1 Corticosterone Production 2 and 4 hours after Acute SEA or Saline Injection
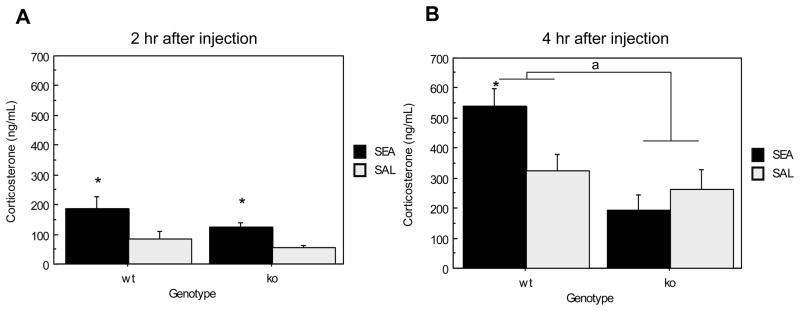
The 2 hr and 4 hr acute experiments were conducted separately and therefore separate ANOVAs were conducted on the data. Two hours following acute injection, plasma corticosterone of SEA treated mice was significantly higher than that of saline injected controls (F(1,20) = 11.350, p=.0031) (See Figure 2A). However, the main effect of genotype was non-significant as corticosterone levels were similarly elevated between genotypes (F(1,20) = 3.150, p= .0912). Figure 2B shows plasma corticosterone 4 hrs after injection. SEA exposure significantly increased plasma corticosterone in ORL1+/+ mice relative to ORL1−/− mice (F(1,18) = 13.264, p=.0019). The main effect of toxin was non-significant (F(1,18) = 1.705, p=.2081) and was likely attributable to the lack of an SEA effect in ORL1−/− mice. However, there was a Genotype × Toxin interaction (F(1,18) = 6.193, p= .0228) indicating that ORL1+/+ mice administered SEA had significantly higher levels of plasma corticosterone than similarly treated, ORL1−/− mice.
Figure 2.
Plasma corticosterone response to SEA 2 and 4 hrs after injection in ORL1+/+ and ORL1−/− mice. (A) 2 hours after injection; (B) 4 hours after injection.
Data are expressed as means ± standard error of the means (SEMs). N = 5–6/gp. *p < .05 relative to corresponding saline. a p < .05 relative to ORL−/− mice.
1.2 Splenic TNFα and IL-2 Production 2 and 4 hours after Acute SEA or Saline Injection
Two hours after acute SEA injection, splenic TNFα was equally elevated in both genotypes (F(1,20) = 413.984, p< .0001) (Table 1). By four hours after acute SEA or saline injection splenic TNFα was significantly lower relative to 2 hr levels, with SEA induced TNFα higher in ORL1+/+ mice than ORL1−/− mice (F(1,18) = 4.315, p=.0524) (Table 1). Splenic IL-2 was dramatically elevated in SEA treated mice relative to saline injected controls at both 2 (F(1,20)= 373.092, p< .0001) and 4 (F(1,18) = 151.167, p< .0001) hours after injection (Table 1). Further, although at 2 hrs there was no difference between genotypes, at 4 hrs SEA-injected ORL1−/− mice had higher splenic IL-2 levels than ORL1+/+ mice (F(1,18)= 6.850, p= .0175).
Table 1.
Effect of SEA Treatment on Splenic IL-2 and TNFα Production (pg/μg protein)
| 2 hrs Acute | 4 hrs Acute | |||
|---|---|---|---|---|
| Group | TNFα | IL-2 | TNFα | IL-2 |
| WT, SEA | 0.387 ± 0.027* | 1.077 ± 0.066* | 0.029 ± 0.003*, b | 0.103 ± 0.013* |
| WT, SAL | 0.012 ± 0.005 | 0.0 ± 0.0 | 0.004 ± 0.001 | 0.002 ± 0.002 |
| KO, SEA | 0.355 ± 0.022* | 1.097 ± 0.089* | 0.021 ± 0.002* | 0.164 ± 0.013*, c |
| KO, SAL | 0.010 ± 0.005 | 0.017 ± 0.015 | 0.002 ± 0.001 | 0.009 ± 0.004 |
p < .05 relative to corresponding saline.
p < .05 relative to KO, SEA.
p < .05 relative to WT, SEA.
1.3 Expression of CRH, TNFα, and TNFR1mRNA in the Hypothalamus and Amygdala of 129S6 ORL1+/+ Receptor Wildtype and ORL1−/− Receptor Knockout Mice
CRH mRNA Expression
Hypothalamus
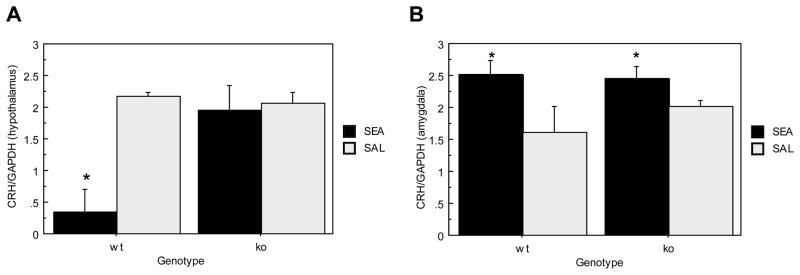
Figure 3A depicts hypothalamic CRH mRNA expression. Analyses revealed main effects of genotype (F(1,18)= 6.122, p=.0235) and toxin (F(1,18)=10.256, p=.0049) as ORL1−/− mice had significantly higher hypothalamic CRH mRNA expression than ORL1+/+ mice. However, this was due to SEA exposure significantly reducing CRH mRNA in ORL1+/+ mice (See Figure 3A), which was suggested by a significant Genotype × Toxin effect (F(1,18)= 3.928, p= .0118).
Figure 3.
Brain CRH mRNA expression in response to acute SEA administration in ORL1+/+ and ORL1−/− mice 4 hours after injection. (A) Hypothalamus; (B) Amygdala. Data are expressed as means ± SEM. N = 5–6/gp. *p < .05 relative to corresponding saline. a p < .05 relative to ORL−/− mice.
Amygdala
Figure 3B shows CRH mRNA expression within the amygdala. There was no difference between ORL1+/+ mice and ORL1−/− mice (F(1,18)<1, p= .5065). However, irrespective of genotype, SEA treated mice had significantly higher CRH mRNA levels than saline treated controls (F(1,18)= 7.565, p= .0132).
TNFα mRNA Expression
Hypothalamus
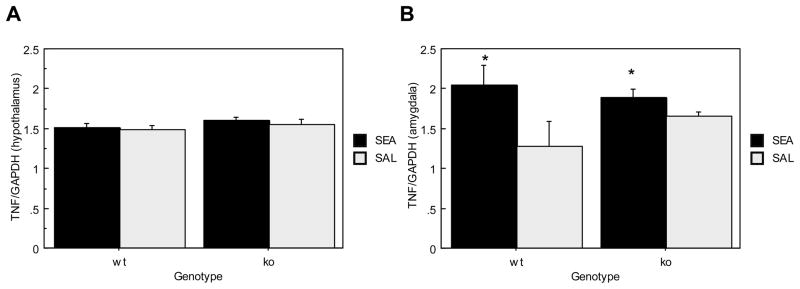
Figure 4A shows hypothalamic TNFα mRNA expression, which did not differ between ORL1+/+ and ORL1−/− mice (F(1,18)= 2.226, p= .1530) nor between SEA and saline treated mice (F(1,18)<1, p= .4344) 4 hrs after acute injection.
Figure 4.
Brain TNFα mRNA expression in ORL1+/+ and ORL1−/− mice 4 hours after acute SEA injection. (A) Hypothalamus; (B) Amygdala. Data are expressed as means ± SEM. N = 5–6/gp. *p < .05 relative to corresponding saline.
Amygdala
Overall TNFα mRNA expression in the amygdala (Figure 4B) did not differ between ORL1+/+ and ORL1−/− mice (F(1,18) < 1, p= .5947), although exposure to SEA significantly increased TNFα mRNA expression (F(1,18)= 5.667, p= .0285). While it appears that TNFα mRNA is higher in ORL1+/+ mice, the Genotype × Toxin interaction was non-significant.
TNFR1 mRNA Expression
Hypothalamus
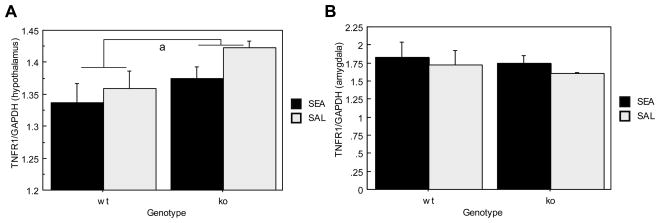
Figure 5A shows hypothalamic TNFR1 mRNA expression, which was significantly lower in ORL1+/+ mice relative to ORL1−/− mice (F(1,18)= 4.681, p= .0442). However, Toxin exposure did not affect TNFR1 mRNA expression (F(1,18)= 2.236, p= .1522) in the hypothalamus.
Figure 5.
Brain TNFα Receptor I mRNA expression in ORL1+/+ and ORL1−/− mice 4 hours after acute SEA injection. (A) Hypothalamus; (B) Amygdala. Data are expressed as means ± SEM. N = 5–6/gp. a p < .05 relative to ORL−/− mice.
Amygdala
Neither main nor interaction effects were observed for amydaloid, TNFR1 mRNA expression (See Figure 5B).
2. Behavioral Effects of Acute SEA Exposure in 129S6 ORL1+/+ Receptor Wildtype and ORL1−/−
Receptor Knockout Mice
2.1 Liquid Diet Consumption 2 and 4 hours Following Acute SEA or Saline Injection
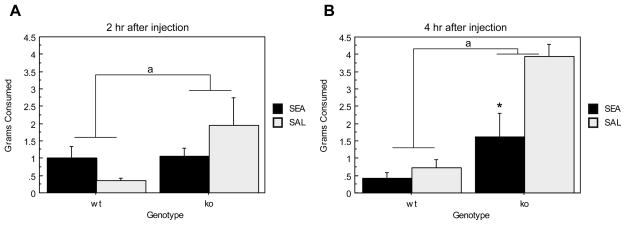
Previous studies have determined that following SEA exposure, elevated levels of plasma corticosterone are accompanied by novelty induced hypophagia (viz., Prosobee; see Materials and Methods) 2 hours after injection (Kawashima and Kusnecov, 2002). Figure 6 shows total Prosobee consumed 2 and 4 hrs after SEA or saline injection for 129S6 ORL1+/+ and ORL1−/− mice. Analyses revealed a main effect of genotype (F(1,17) = 25.983, p< .0001) indicating that ORL1−/− mice consumed significantly more Prosobee than ORL1+/+ mice. Moreover, a main effect of Toxin was found as SEA-injected mice consumed significantly less Prosobee than saline treated mice (F(1,17) = 5.769, p=.0280), a finding that is largely attributable to the SEA-induced anorexia evident 4 hrs after injection (Time × Toxin interaction: F(1,17) = 5.813, p= .0275). Finally, there was a significant Genotype × Toxin interaction (F(1,17) = 9.071, p=.0079), suggesting that SEA treatment differentially affected the consumption of wildtype and knockout mice. As seen in Figure 6, it is clear that SEA-induced reduction in consumption is more strongly evident in ORL1−/− mice for both time points. However, SEA increased consumption by ORL1+/+ mice at 2 hrs, but this subsequently declined below saline-treated mice by 4 hrs (See Figure 6). Overall, irrespective of treatment, consumption was greater in ORL1−/− mice across both time points.
Figure 6.
Consumption of a novel liquid diet in ORL1+/+ and ORL1−/− mice given a single injection of SEA or saline. (A) 2 hours after injection; (B) 4 hours after injection.
Data are expressed as means ± standard error of the means (SEMs). N = 4–6/gp. *p < .05 relative to corresponding saline. a p < .05 relative to ORL−/− mice.
2.2 Open Field and Novel Object Test 4 Days Following Acute SEA or Saline Injection
Four days following SEA or saline injection, and consumption testing, exploration was measured in the open field apparatus prior to and immediately after presentation of a novel object. Specific behaviors measured are listed in the materials and methods section.
Open Field Test
Prior to presentation of the novel object, groups did not differ in percentage time spent in latency to enter or number of entries made into the center of an open field. Further, groups did not differ in percent time spent in the periphery. Thus, neither genotype nor exposure to SEA influenced behavior during open field testing prior to introduction of a novel object.
Novel Object Test
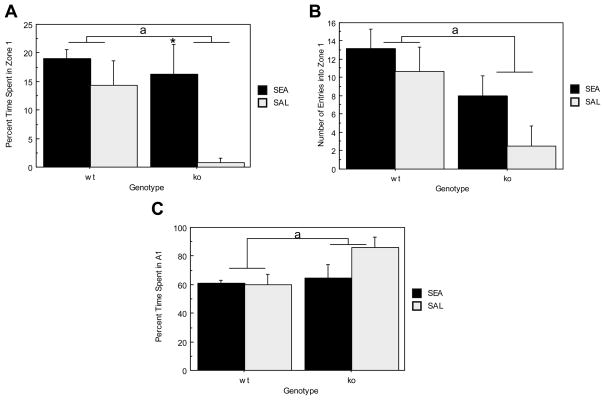
Figure 7A depicts percent time spent in the center of an open field (zone 1; see figure 1A) upon introduction of a novel object. Specifically, ORL1+/+ mice spent significantly more time in zone 1 than ORL1−/− mice (F(1, 17)= 4.851, p= .0417) suggesting that ORL1−/− mice are more anxious. Moreover, although ANOVA revealed that SEA pretreated mice spent significantly more time in zone 1 than did saline pretreated controls (F(1, 17)= 7.568, p= .0136), this effect was due to the dramatic difference between SEA and saline pretreated ORL1−/− mice (see Figure 7A). Interestingly, latency to first enter zone 1 did not differ between genotypes (F(1, 17)= 2.966, p= .1032) and was not affected by acute SEA exposure 4 days prior to testing (F(1, 17)= 1.705, p=.2090). Figure 7B illustrates the number of entries made into zone 1 following introduction of the novel object. Specifically, ORL1+/+ mice made significantly more entries into the center than did ORL1−/− mice (F(1, 17)= 7.548, p=.0137). Again this was likely due to the low number of entries made by ORL1−/−, saline-pretreated mice (see Figure 7B).
Figure 7.
Exploratory behavior in the open field in the presence of a novel object. (A) Percent time in zone 1 (see Figure 2 for schematic); (B) Number of entries into zone 1; (C) percent time in periphery (area A1, see Fig. 2). Data are expressed as means ± standard error of the means (SEMs). N = 4–6/gp. *p < .05 relative to corresponding saline. a p < .05 relative to ORL−/− mice.
Figure 7C shows the percent time spent in the periphery during novel object testing. A significant effect of genotype was found indicating that ORL1−/− mice spent significantly more time in the periphery of the open field than ORL1+/+ mice (F(1, 17)= 4.824, p= .0422). Thus, although groups did not differ in their latency to first approach the novel object, an anxiogenic like phenotype was evident in ORL1−/− mice. Specifically, ORL1−/− mice displayed increased thigmotaxic behavior as they made significantly fewer entries into zone 1 during novel object testing and spent significantly less time in the center of the open field than ORL1+/+ mice. However, SEA pretreatment four days prior to novel object testing increased percent time spent in the center mainly for ORL1−/− mice.
Number of Interactions with the Novel Object
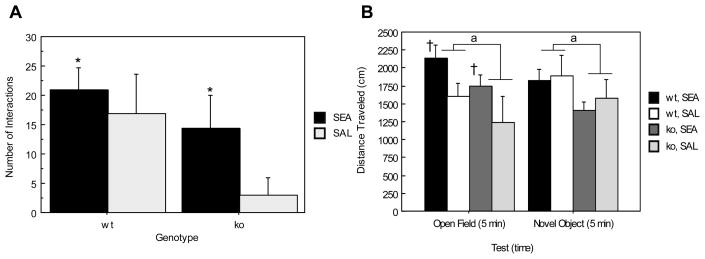
ANOVA revealed a main effect of toxin such that SEA-pretreated animals interacted significantly more with the novel object than animals administered saline (F(1,16)= 4.979, p= .0403) (Figure 8A). Although ORL1+/+ and ORL1−/− mice did not differ in the number of interactions made with the novel object (F(1,16)= 2.659, p=.1225) it is evident from Figure 8A that this is likely due to the SEA-induced reversal of anxiogenic-like behavior observed in saline pretreated, ORL1−/− mice.
Figure 8.
Physical activity and novel object interactions. (A) Number of interactions (rearings and contacts) with the novel object; (B) locomotor activity prior to and during novel object presentation. Data are expressed as means ± standard error of the means (SEMs). N = 4–6/gp. *p < .05 relative to corresponding saline. a p < .05 relative to ORL−/− mice. †p < .05 relative to saline during open field testing only.
Total Distance Traveled During Open Field and Novel Object Testing
Figure 8B depicts total distance traveled during open field and novel object testing. A significant effect of genotype was found indicating that ORL1+/+ mice exhibited significantly higher levels of locomotor activity than did ORL1−/− mice (F(1, 17)= 5.729, p= .0285). Moreover, statistical analyses revealed a significant Test × Toxin interaction such that animals administered SEA exhibited significantly higher levels of locomotor activity than saline treated controls during open field testing (F(1, 17)= 4.538, p= .0481) but not during novel object testing. Therefore, the increased anxiogenic like behavior evident in ORL1−/− receptor knockout mice during novel object testing cannot be attributed to decreased locomotor activity.
4. Discussion
The present study tested whether exposure of 129S6, ORL1−/− mice to an immunologic stressor, the T cell superantigen SEA, alters various parameters of endocrine, behavioral and cytokine responsiveness. A prominent feature of SEA challenge is activation of the HPA axis, with elevations of plasma corticosterone being CRH-dependent (Kaneta, 2005; Rossi-George, 2005). In the present study, two hours following SEA challenge, plasma corticosterone was significantly elevated in both wildtype and ORL1 knockout mice; however, four hours after challenge, ORL1−/− mice did not differ from saline-injected controls. Therefore, under conditions of acute immunologic challenge, the ORL1 gene appears to confer prolonged stimulation of the HPA axis. This conclusion is in keeping with other evidence that intracerebroventricular (icv) administration of OFQ/N produces an exaggerated and prolonged stress-induced elevation in plasma corticosterone (Devine et al., 2001; Fernandez et al., 2004).
The neuropeptide CRH is a primary ACTH secretagogue, and was shown to be responsible for HPA axis activation in response to various T cell superantigens, including SEA (Kusnecov et al., 1999; Rossi-George et al., 2005). To determine whether the corticosterone responses to SEA in ORL1+/+ and ORL1−/− mice were associated with variations in CRH mRNA, real-time PCR was conducted on the hypothalamus and amygdala, two areas that contain abundant quantities of CRH. The four hour time point was chosen, since previously it was observed that CRH mRNA changes in the paraventricular nucleus of the hypothalamus do not occur 2 hours after T cell activation with superantigens (Kusnecov et al, 1999). In both brain regions examined, CRH mRNA levels did not differ between the two genotypes in response to saline injections. However, following SEA challenge hypothalamic CRH mRNA expression was dramatically reduced in ORL1+/+ mice, but did not change in ORL1−/− mice. Given that hypothalamic expression of ppOFQ/N mRNA increased following SEA injection (Kawashima and Kusnecov, 2002), and icv administration of OFQ/N augmented and prolonged HPA axis activation (Devine et al., 2001; Fernandez et al., 2004), the present findings suggest that SEA administration results in utilization of hypothalamic CRH mRNA in an ORL1 dependent manner. Consequently, it could be hypothesized that after primary SEA exposure, the presence of ORL1 signaling is associated with prolonged stress-induced levels of corticosterone by way of augmented CRH-dependent activity in the hypothalamus.
An alternative conceptualization is that the reduced CRH mRNA levels in SEA-treated ORL-1+/+ mice reflected increased glucocorticoid-mediated repression of the CRH gene, a phenomenon that has been well documented (van der Laan et al., 2008). This is conceivable in light of the fact that in ORL-1−/− mice SEA-treatment did not significantly change the level of hypothalamic CRH mRNA at 4 hrs, which is a time point that coincided with significantly lower corticosterone levels (and hence less negative feedback) than that observed in SEA-treated ORL-1+/+ mice. Consequently, the lower hypothalamic CRH mRNA in the latter animals may have been due to glucocorticoid-mediated repression of the CRH gene at the 4 hr time point. Interestingly, for the amygdala, CRH mRNA was increased in response to SEA challenge, although this did not appear to rely on the presence of the ORL1 gene. Nonetheless, it was consistent with other data for increased CRH mRNA in the central nucleus of the amygdala after superantigen challenge (Kusnecov et al, 1999).
Measures were also taken of hypothalamic and amygdaloid, TNFα mRNA expression, since previously in C57BL6/J mice the neural, endocrine and behavioral effects of SEA were shown to be dependent on TNFα (Rossi-George et al, 2005). Systemically, the 129S6 background mice tested in the current study showed normal splenic TNFα (and IL-2) responses to SEA. Most importantly, however, brain TNFα mRNA was also increased in the amygdala, but not hypothalamus, in SEA challenged mice. This occurred for both wildtype and knockout mice, ruling out the possibility that ORL1 is necessary for modulation of central TNFα mRNA levels by immunologic challenge with SEA. It is of interest to note, however, that CRH has been shown to promote TNFα production in mouse macrophages in vitro and in vivo (Agelaki et al., 2002). Considering both the CRH and TNFα mRNA data for the amygdala, it could be hypothesized that amygdaloid CRH may influence TNFα production following a systemic immunologic challenge. Moreover, the source of central TNFα production may actually be of microglial origin. These cells are derived from the same myeloid lineage as macrophages, perform phagocytic functions in the brain, and produce TNFα (Lambertsen et al., 2009; Mott et al., 2004). Given that CRH is capable of enhancing the production of TNFα from macrophages, it is conceivable that central TNFα production may be stimulated by CRH.
Many of the major neurobiological effects of TNFα, such as HPA axis activation and hypophagia, are mediated via the TNFRI receptor (Rossi-George et al., 2005). In spite of the SEA-induced variations in amygdaloid TNFα mRNA, corresponding changes in TNFR1 mRNA were not observed in the amygdala, or in the hypothalamus. However, overall TNFRI mRNA in the hypothalamus was higher in ORL1−/− mice, suggesting that ORL1 signaling may be associated with basal TNFα cytokine signaling in the hypothalamus. Interestingly, although there is little information on the relationship between the OFQ/N system and TNFα in the brain, there is in vitro evidence showing that astrocyte activation of the OFQ/N precursor gene is enhanced by TNFα (Buzas et al., 2002). In view of this evidence, at the very least, the present data support the notion of a potential functional relationship between TNFα, TNFα receptors and the OFQ/N system in the hypothalamus that awaits further investigation.
The current study also investigated the behavioral effects of immunologic challenge with SEA. This involved testing for consummatory effects 2 and 4 hrs after SEA challenge, with further testing for exploratory behavior four days later. The decision to test four days after injection was based on previous evidence showing that in vivo the proliferative phase of SEA-activated T cells peaks at 2 days and subsides by 4 days after a single injection of SEA (Chen et al., 2002; McCormack et al., 1993).
It has already been demonstrated in other strains of mice (viz., BALB/c and C57BL/6) that T cell superantigens reduce intake of a novel liquid diet (Kusnecov and Goldfarb, 2005), raising the hypothesis of augmented gustatory neophobia, which was later shown to be TNFα dependent (Rossi-George et al., 2005). In the current study, it was notable that after SEA treatment, 129S6 wildtype mice did not show a major reduction in consumption of the novel liquid diet. As noted earlier, this was not due to a failure of mice to respond with TNFα and IL-2 production to SEA. Additional experiments in our lab confirmed that the 129S6 strain differs significantly from C57BL/6 mice in terms of the hypophagic effect of SEA using the Prosobee test. That is, as previously reported (Rossi-George et al., 2005; Urbach-Ross et al., 2008), 2 hrs after 5 μg SEA challenge C57BL/6 mice showed 60% reduction in consumption, while 129S6 mice fail to show a significant reduction (unpublished data). Therefore, we are confident that the 129S6 mouse strain is resistant to the anorexic effects of SEA under the given conditions of consumption testing.
Of considerable interest, however, is that ORL1 deletion produces significant effects on consumption of the liquid diet. For instance, saline-injected ORL-1−/− mice showed higher levels of consumption relative to ORL-1+/+ mice similarly injected with saline. Therefore, deletion of the ORL-1 gene appeared to produce an orexigenic effect. This may indeed have conferred greater sensitivity to the anorexic effects of SEA, that otherwise was lacking in ORL-1+/+ 129S6 mice. These observations suggest that intact OFQ/N signaling in the 129S6 mouse may confer resistance or reduced sensitivity to anorexic signals generated in response to SEA challenge. Since in the C57BL/6 mouse, deletion of TNFα or in vivo neutralization of circulating TNFα abolishes the anorexic response to SEA (Rossi-George et al., 2005), it is possible that the anorexigenic effect of endogenous TNFα is weak in 129S6 mice, and that this relies on the presence of a functional ORL1 gene. Indeed, it is interesting to note that as discussed above, TNFRI mRNA in the hypothalamus was higher in the ORL1 knockout mice. Therefore, given that the hypothalamus is central to the regulation of food intake (Cowley, 2003; Shioda et al., 2008), it is possible that the presence of the ORL1 gene in the hypothalamus down-regulates TNFRI expression, and renders 129S6 mice less sensitive to the anorexigenic effects of TNFα.
In addition to gustatory behavior, animals were tested on the fourth day after injection with SEA for exploration of an open field in the absence and presence of a novel object. This test allows for a general measure of locomotor behavior in a novel environment (open field), as well as stimulus-specific reactions to novelty (neophobia in response to a novel object) (Kawashima and Kusnecov, 2002). Neither SEA pre-exposure nor genotype influenced behavior during open field testing. The lack of an SEA effect in the open field was consistent with previous observations using C57BL/6 mice (Kawashima and Kusnecov, 2002). However, behaviors measured in the presence of the novel object differed between ORL1+/+ mice and ORL1−/− mice and between animals administered SEA or saline. As reported in the results, saline-treated ORL1+/+ mice showed greater exploration of central areas of the open field that contained the novel object, which is consistent with previous studies showing that the loss of OFQ/N-ORL1 signaling results in increased anxiogenic-like behavior (Gavioli et al., 2007; Griebel et al., 1999; Jenck et al., 1997; Jenck et al., 2000; Koster et al., 1999). Additional measures of latency and interactions with the novel object indicated greater anxiety-like behavior in the ORL1−/− mice that had been pretreated with saline. Interestingly, while SEA pretreatment four days earlier did not affect behavior of wildtype mice, the ORL1−/− mice appeared to show reduced signs of anxiety-like behavior, and increased exploration of the novel object, as measured by percent time spent in the immediate region around the novel object.
Furthermore, while actual physical interactions with the novel object increased if both genotypes were pretreated with SEA, the magnitude change appeared to be greater for the ORL1−/− mice pretreated with SEA (i.e. five-fold increase compared to two-fold increase in wildtype mice). These data are consistent with the notion that behavioral effects after T cell activation with SEA are more prominent if the ORL1 gene is deleted, as was discussed for the consumption data. The nature of the change varies with the type of test and temporal distance from SEA challenge. Additional experimentation is required to determine whether open field testing shortly after SEA challenge will result in a reversal of the anxiety-like behavior of ORL1−/− mice. For example, two hours after SEA challenge, wildtype C57BL/6 mice show less interaction with a novel object (Kawashima and Kusnecov, 2002), although behavior in traditional tests of anxiety, such as the elevated plus maze and light-dark box revealed a non-anxiogenic profile characterized by greater exploration (Rossi-George et al., 2004). It is possible that SEA challenge, as an immunologic stressor that activates the HPA axis, augments heightened arousal and or attention-related mechanisms that modify responses to biologically meaningful stimuli (e.g., novel foods and environmental contexts). Moreover, this influence appears to be enhanced by deletion of the ORL1 gene.
The current study measured concentrations of TNF and IL-2 in spleens collected 2 and 4 hours after administration of SEA. This was conducted to ensure that SEA challenge in 129/S6 mice engaged T cells. As expected, SEA produced significant elevations in both cytokines at these time points. Interestingly, at the 2 hr time point, these elevations were similar in both genotypes, suggesting that ORL-1 deletion was not important in the early production of TNF and IL-2 induced by SEA. At the 4 hr time point, TNF concentrations had declined substantially, but were still slightly higher in wildtype mice. Although significant, this amounted to only a modest difference unlikely to account for the corticosterone differences between SEA-treated wildtype and knockout mice at the 4 hr time point. Interestingly, IL-2 levels at 4 hrs after injection were significantly lower in wildtype mice, suggesting that downregulation of IL-2 production requires ORL-1 signaling. These cytokine data stand in contrast to our previous finding in mice with a deletion in the precursor for OFQ/N, which had suggested that endogenous OFQ/N levels are necessary to ensure optimal levels of TNFα production 2hrs after SEA challenge (Goldfarb et al, 2006). However, in the present study, the failure to see relatively less 2 hr splenic TNFα production in ORL-1−/− mice challenged with SEA might be due to differences in the background genetic strain (129/S6 vs C57BL/6), and is a question requiring further investigation. Moreover, the 4 hr data (a time point not tested by Goldfarb et al) suggests that endogenous OFQ/N signaling might be necessary for preserving sufficient amounts of TNFα as the rate of synthesis declines, while promoting downregulation of IL-2 production. Since the balance of TNF and IL-2 production are features evident in neuroimmunopathological conditions, including multiple sclerosis (Shi et al, 2009), it will be important to explore the role of OFQ/N signaling in immunoregulation.
In conclusion, the findings of the present study demonstrate a role for ORL1 in the modulation of immunological, endocrine and behavioral functioning following systemic challenge with SEA. Specifically, subsequent to SEA exposure, intact OFQ/N- ORL1 signaling prolonged stress-induced stimulation of the HPA axis through ORL1 dependent modulation of hypothalamic, CRH mRNA expression. Consistent with previous reports, removal of the ORL1 gene produced an anxiogenic-like phenotype during behavioral tests of anxiety and gustatory neophobia. Unexpectedly, pretreatment with SEA, four days prior to testing reversed the anxiogenic-like response of ORL1−/− mice during novel object testing. Although the endocrine and behavioral effects of SEA have previously been shown to be TNFα dependent in C57BL/6J mice, elucidation of the relationship between ORL1 and modulation of TNFα dependent processes following SEA has proven difficult in ORL1 transgenic mice generated on a 129S6 background. Nonetheless, the present data confirm that 129S6 mice are immunologically responsive to SEA and suggest a role for ORL1 in basal TNFα signaling, modulation of HPA activity and the behavioral response to systemic immune challenge with SEA.
Acknowledgments
Supported by grants MH60706 and NIEHS P30 ES05022
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Buzas B, Rosenberger J, Kim KW, Cox BM. Inflammatory mediators increase the expression of nociceptin/orphanin FQ in rat astrocytes in culture. Glia. 2002;39:237–246. doi: 10.1002/glia.10106. [DOI] [PubMed] [Google Scholar]
- Chen L, Koyanagi M, Fukada K, Imanishi K, Yagi J, Kato H, Miyoshi-Akiyama T, Zhang R, Miwa K, Uchiyama T. Continuous exposure of mice to superantigenic toxins induces a high-level protracted expansion and an immunological memory in the toxin-reactive CD4+ T cells. J Immunol. 2002;168:3817–3824. doi: 10.4049/jimmunol.168.8.3817. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M. Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav. 2004;82:63–68. doi: 10.1016/j.physbeh.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Cooper JF, Kusnecov AW. Methylmercuric chloride induces activation of neuronal stress circuitry and alters exploratory behavior in the mouse. Neuroscience. 2007;148:1048–1064. doi: 10.1016/j.neuroscience.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA. Hypothalamic melanocortin neurons integrate signals of energy state. Eur J Pharmacol. 2003;480:3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Watson SJ, Akil H. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neuroscience. 2001;102:541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Gavioli EC, Rizzi A, Marzola G, Zucchini S, Regoli D, Calo G. Altered anxiety-related behavior in nociceptin/orphanin FQ receptor gene knockout mice. Peptides. 2007;28:1229–1239. doi: 10.1016/j.peptides.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Orphanin FQ, a novel neuropeptide with anti-stress-like activity. Brain Res. 1999;836:221–224. doi: 10.1016/s0006-8993(99)01684-4. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneta T, Kusnecov AW. The role of central corticotropin-releasing hormone in the anorexic and endocrine effects of the bacterial T cell superantigen, Staphylococcal enterotoxin A. Brain Behav Immun. 2005;19:138–146. doi: 10.1016/j.bbi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Fugate J, Kusnecov AW. Immunological challenge modulates brain orphanin FQ/nociceptin and nociceptive behavior. Brain Res. 2002;949:71–78. doi: 10.1016/s0006-8993(02)02966-9. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol. 2002;123:41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- Kohman RACB, Urbach-Ross D, Kusnecov AW. Influence of age on behavioral, immune, and endocrine responses to the T-cell superantigen staphylococcal enterotoxin A. doi: 10.1111/j.1460-9568.2009.06921.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnecov AW, Goldfarb Y. Neural and behavioral responses to systemic immunologic stimuli: a consideration of bacterial T cell superantigens. Curr Pharm Des. 2005;11:1039–1046. doi: 10.2174/1381612053381602. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Liang R, Shurin G. T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J Neurosci. 1999;19:4533–4543. doi: 10.1523/JNEUROSCI.19-11-04533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JE, Callahan JE, Kappler J, Marrack PC. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mollereau C, Mouledous L, Lapalu S, Cambois G, Moisand C, Butour JL, Meunier JC. Distinct mechanisms for activation of the opioid receptor-like 1 and kappa-opioid receptors by nociceptin and dynorphin A. Mol Pharmacol. 1999;55:324–331. doi: 10.1124/mol.55.2.324. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Jr, Civelli O. Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J Biol Chem. 1996;271:14163–14168. doi: 10.1074/jbc.271.24.14163. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, LeBlanc F, Kaneta T, Urbach D, Kusnecov AW. Effects of bacterial superantigens on behavior of mice in the elevated plus maze and light-dark box. Brain Behav Immun. 2004;18:46–54. doi: 10.1016/s0889-1591(03)00087-4. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Takenoya F, Yagi M, Wang L, Hori Y, Kageyama H. Neural networks of several novel neuropeptides involved in feeding regulation. Nutrition. 2008;24:848–853. doi: 10.1016/j.nut.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Urbach-Ross D, Crowell B, Kusnecov AW. Relationship of varying patterns of cytokine production to the anorexic and neuroendocrine effects of repeated Staphylococcal enterotoxin A exposure. J Neuroimmunol. 2008;196:49–59. doi: 10.1016/j.jneuroim.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Calo G. Nociceptin/orphanin FQ and its receptor--potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–429. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]