Abstract
Dendritic cells (DCs) are often exposed to various oxygen tensions under physiological and pathological conditions. However, the effects of various oxygen tensions on DC functions remain unclear. In this study, we showed that hypoxia-differentiated DCs expressed lower levels of MHC-II molecule, co-stimulatory molecules (CD80, CD86) and proinflammatory cytokines (IL-1β, IL-6, and TNF-α), but higher levels of immunoregulatory cytokine transforming growth factor-beta (TGF-β) than normoxia-differentiated DCs. Unexpectedly, re-exposure of hypoxia-differentiated DCs to saturated oxygen (reoxygenation) completely restored their mature phenotype and function. Specifically, the reoxygenated DCs induced naïve CD4+ T cells to differentiate into Th1 and Th17 effector cells, but deceased the generation of CD4+CD25+Foxp3+ regulatory T cells (Tregs). The data indicate that hypoxic microenvironment suppresses the maturation and function of murine DCs. Reoxygenation of hypoxia-differentiated DCs however results in complete recovery of their mature phenotype and function, and has strong ability to drive immune response toward a proinflammatory direction, suggesting reoxygenated DCs may contribute to inflammation of ischemia-reperfusion injury.
Keywords: Dendritic cells, Inflammation, Foxp3+ Tregs, IL-6, TGF-β, Hypoxia
1. Introduction
Hypoxia exists in many ischemia conditions such as organ transplantation, trauma, hypovolemic shock, liver surgery and cardiovascular diseases, the following return of blood flow and oxygen delivery in the hypoxic tissues lead to rapid change of oxygen tension, which often exacerbates the damage of hypoxic organ and induces ischemia-reperfusion injury (IRI) (Kutala et al., 2007; Li and Jackson, 2002; Wang et al., 2002; Yamauchi and Kimura, 2008). There are considerable data suggesting an important role of innate and adaptive immune response in IRI (Boros and Bromberg, 2006). Dendritic cells (DCs), the professional antigen presenting cells (APCs), are the initiator to drive adaptive immune responses. Recent studies have provided novel insights into the potential role of DCs in mediating immune responses in IRI. Accumulation of DCs was observed in liver and kidney within 1 h and peaked at 24 h after IRI (Zhou et al., 2005). Increased number of DCs expressing more mature phenotype were identified in ischemic kidney in the early phase (within 24 h) after IRI, while these activated DCs with a strong capacity of inducing T cell proliferation migrated from the kidney into the renal lymph node 1 day after IRI (Dong et al., 2005). DCs isolated from liver after IRI also exhibited a mature phenotype (Loi et al., 2004). These data suggest that activated DCs are involved in IRI. However, the mechanism of DC activation during IRI is largely unknown.
It has been suggested that reoxygenation (re-exposure to saturated oxygen) after hypoxia is a crucial step mediating organ injury in IRI, where oxygen availability leads to a dramatic shift of oxygen tension (Cao et al., 2006; Leonard et al., 2006; Li and Jackson, 2002; Saikumar et al., 1998; Wang et al., 2002). Previous studies have demonstrated that hypoxia inhibits the activation and cytokine secretion of CD4+ T cells (Caldwell et al., 2001), but increases the phagocytosis of macrophages (Anand et al., 2007). Several studies have indicated that hypoxia affects differentiation, maturation (Jantsch et al., 2008; Mancino et al., 2008), migration (Zhao et al., 2005; Qu et al., 2005; Yang et al., 2006; Zhao et al., 2008), antigen uptake of DCs (Elia et al., 2008), and their capability to promote adaptive immunity (Jantsch et al., 2008; Mancino et al., 2008). In addition, hypoxia significantly alters the expression profile of cytokines, chemokines and chemokine receptors (Bosco et al., 2008; Elia et al., 2008; Mancino et al., 2008; Ricciardi et al., 2008). However, the conclusions from different reports are controversial. Furthermore, previous researches have failed to consider the impact of reoxygenation on hypoxia-differentiated DCs.
Although the direct or indirect role of CD4+ effector T cells in IRI has been investigated (Huang et al., 2007; Marques et al., 2006), which subsets of CD4+ T cells involved in is not fully understood. Th17 cells and regulatory T cells (Tregs) have been described as two new subsets of CD4+ T cells in addition to Th1 and Th2 types (Bettelli et al., 2006; Harrington et al., 2006; Mosmann et al., 1986; Sakaguchi, 2004; Sakaguchi et al., 1995). Th17 cells producing IL-17 mediate host defensive mechanisms to extracellular bacteria infections, and are involved in the pathogenesis of several organ specific autoimmune diseases which have historically been associated with Th1 responses (Afzali et al., 2007; Korn et al., 2007). Tregs expressing CD25 and Foxp3 have an anti-inflammatory role and maintain tolerance to self components by contact-dependent suppression or releasing anti-inflammatory cytokines such as IL-10 and TGF-β (Chen et al., 2003; Rudensky and Campbell, 2006; Sakaguchi, 2003; Sakaguchi, 2004; Sakaguchi et al., 1995; von Boehmer, 2005; Xiao et al., 2008). DCs may drive either Th effector cell differentiation or induce Treg generation upon the different microenvironment and cytokine milieu (Belkaid and Oldenhove, 2008; Luo et al., 2007; Tang et al., 2006). It remains largely unknown however whether diverse oxygen tension influences the ability of DCs in T cell differentiation.
In this study, we investigated the phenotype and function of murine BM-DCs generated under hypoxic or reoxygenated conditions. We demonstrated that hypoxic microenvironment suppressed the maturation and function of DCs. Reoxygenation of hypoxia-differentiated DCs resulted in complete recovery of their mature phenotype and function, and drove them to initiate Th1 and Th17 cell differentiation, suggesting that DCs play a critical role in inflammation of IRI.
2. Materials and Methods
2.1. Mice
Female BALB/cH-2d and C57BL/6H-2b mice (6-8 weeks) used in the experiments were purchased from Shanghai SLAC Laboratory Animal co. Ltd (Shanghai, China) and maintained in the specific pathogen-free animal facility at Shandong University (Jinan, China). All animal studies were approved by the Animal Care and Utilization Committee of Shandong University, China.
2.2. Reagents and antibodies
Recombinant murine GM-CSF and IL-4 were obtained from PeproTech Inc (New Jersey, USA). FITC-conjugated anti-mouse CD11c, PE-conjugated anti-mouse MHC class II, CD80, CD86, Foxp3, IL-4, IL-17A mAbs, purified anti-mouse CD3 mAb, and Brefeldin A (BFA) were purchased from eBioscience (San Diego, CA, USA). PE-Cy5-conjugated anti-mouse CD4, FITC-conjugated anti-mouse CD25, IFN-γ mAbs were from BD Biosciences (San Jose, CA, USA). Lipopolysaccharides (LPS), Ionomycin and phorbol myristate acetate (PMA) were purchased from Sigma-Aldrich (Saint Louis, USA). Mouse CD4 MicroBeads were obtained from Miltenyi Biotec (Auburn, CA, USA).
2.3. Generation of bone marrow-derived DCs
DCs were generated from the bone marrow (BM) precursors of C57BL/6 mice as described in references (Inaba et al., 1992; Lutz et al., 1999). In brief, BM cells were flushed from femurs and tibias with RPMI 1640 medium and treated with RBC lysis buffer for 5 min, washed twice with phosphate buffered saline (PBS), then cultured in complete RPMI 1640 medium supplemented with 50 ng/ml GM-CSF and 20 ng/ml IL-4. Half of the medium was replaced at day 3 and 5. At day 7, the suspended and loosely adherent cells were collected as immature DCs (CD11c+ cells were about 80%). To induce maturation, the immature DCs were stimulated with LPS (1μg/ml) for additional 24 h. Cells showed DC morphology and phenotype as determined by light microscopy and Flow Cytometry (FCM), respectively. For normoxic culture condition, cells were maintained in a humidified incubator (HERAcell, Germany) containing 21% O2, 5% CO2, 74% N2 at 37 °C. For hypoxic culture condition, cells were incubated in a humidified hypoxic incubator (HERAcell, Germany) flushed with a gas mixture of 1% O2, 5% CO2, and 94% N2 at 37 °C. To ensure that cells was exposed to hypoxic condition, the medium for hypoxic culture was balanced in hypoxic condition at least for 24 h and the time for replacing medium was kept as short as possible. In some experiments, hypoxia-differentiated DCs were transferred from hypoxia to normoxia for different period of time to allow them reoxygenation (Wang et al., 2002). To avoid the effect of various oxygen tensions on phenotype of DCs, cells were fixed before staining. In addition, to indicate the status of hypoxia or normoxia, the expression of hypoxia induced factor-1 alpha (HIF-1α), a major regulator for cell adaptation to hypoxia, was detected by Western blot.
2.4. RT-PCR
Total cellular RNA was extracted from cells using a modified Trizol one-step extraction method, and reverse-transcribed into cDNA using Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s protocol. PCR was performed using specific primers. Primers for amplification of each gene are as follows: IL-1β, sense: 5’-GCA ACT GTT CCT GAA CTC A-3’, antisense: 5’-CTC GGA GCC TGT AGT GCA G-3’; IL-6, sense: 5’-TTC TTG GGA CTG ATG CTG-3’, antisense: 5’-CTG GCT TTG TCT TTC TTG TT-3’; TNF-α, sense: 5’- ATG AGC ACA GAA AGC ATG ATC-3’; antisense: 5’- TAC AGG CTT GTC ACT CGA ATT -3’; MMP9, sense: 5’-ACC CTG TGT GTT CCC GTT-3’; antisense: 5’-CCG TCT ATG TCG TCT TTA TTC A-3’; TGF-β1, sense: 5’- GGC GGT GCT CGC TTT GTA- 3’, antisense: 5’- CGT GGA GTT TGT TAT CTT TGC T-3’; β-actin, sense: 5’-TGC GTG ACA TCA AAG AGA AG-3’, antisense: 5’-TCC ATA CCC AAG AAG GAA GG-3’. RT-PCR was performed at least three independent experiments, mRNA levels for different genes were normalized to the house keeping genes, β-actin using Quantity One® Version 4.3.1 (Bio-Rad, Hercules, CA, USA).
2.5. T cell proliferation assay
CD4+ T cells from the spleen of C57BL/6H-2b or BALB/cH-2d mice were isolated via positive selection by Mouse CD4 MicroBeads as the manufacturer’s protocols. The purity of CD4+ T cells was >95%. To assay the proliferation of CD4+ T cells, DCs were induced from precursors in BM of C57BL/6 H-2b mice in presence of IL-4 and GM-CSF for 5-7 days in hypoxia, stimulated by LPS for maturation for 18h in hypoxia and then these dendritic cells were divided into two groups: one was transferred into normoxia for additional 6 hours, describes as reoxygenated DCs (H/R), the other was still kept in hypoxia for additional 6 hours, named as hypoxic DCs (H). Meanwhile, normoxia-differentiated DCs (N) were used as control. After treated with mitomycin C (Mancino et al., 2008). DCs were co-cultured with CFSE (Molecular Probes, Inc. Eugene, OR, USA)-labeled CD4+ T cells from C57BL/6H-2b or BALB/cH-2d mice at a ratio of 1:10 in 24-well plates in the presence or absence of anti-CD3 mAb (0.5 μg/ml). The proliferation rate was measured via FCM at day 3 or 5. In some experiments, mitomycin C-treated DCs from C57BL/6H-2b mice were co-cultured with CD4+ T cells from BALB/cH-2d mice for 5 days (MLR) as described above, intracellular cytokines were determined by FCM.
2.6. Flow cytometry
For the staining of surface molecules, DCs were fixed with 1% paraformaldehyde, followed by staining with FITC-conjugated anti-CD11c, PE-conjugated anti-MHC class II, CD80, and CD86 mAbs. For the detection of intracellular cytokines, the cells were stimulated by Ionomycin (1 μg/ml) and PMA (50 ng/ml) for 5 h, BFA (1:1000) was added for the final 2 h, followed by staining for surface CD4 and intracellular IFN-γ, IL-4 and IL-17A. For the assay of CD4+CD25+Foxp3+ Tregs, the cells were stained with PE-Cy5-conjugated anti-CD4 and FITC-conjugated anti-CD25 mAbs, then fixed and permeabilized using Foxp3 staining buffer set (eBioscience, San Diego, CA, USA) followed by Foxp3 staining. FCM data acquisition and analysis were performed on a FACS Calibur Flow Cytometer and FACS research soft CellQuest software (BD Biosciences, Mountain view, CA, USA).
2.7. ELISA
Supernatants from DCs or MLR culture systems were harvested and analyzed for the production of cytokines IL-1β, IL-6, TNF-α, IL-4, IFN-γ, and TGF-β using commercial ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s protocols. A standard curve was generated using recombinant cytokine for each assay.
2.8. Western blot
DCs generated under different conditions were lysed using lysis buffer, and protein concentration was determined by Bradford kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein extracts were separated by SDS-PAGE and transferred onto PVDF membranes. After blocking with 5% non-fat milk for 1 h, the membranes were incubated overnight at 4°C with anti-HIF-1α (1:500) or anti-β-actin (1:2000) Abs (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then incubated with the corresponding secondary antibodies at room temperature for 1 h. After washing, the protein bands were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
2.9. Statistical analysis
The student’s t test or ANOVA was used to assess the significant differences between groups with SPSS 11.5 software. P<0.05 was considered statistically significant. *, P<0.05; **, P<0.01. ***, P<0.001.
3. Results
3.1. Hypoxia inhibits the phenotype and function of DCs
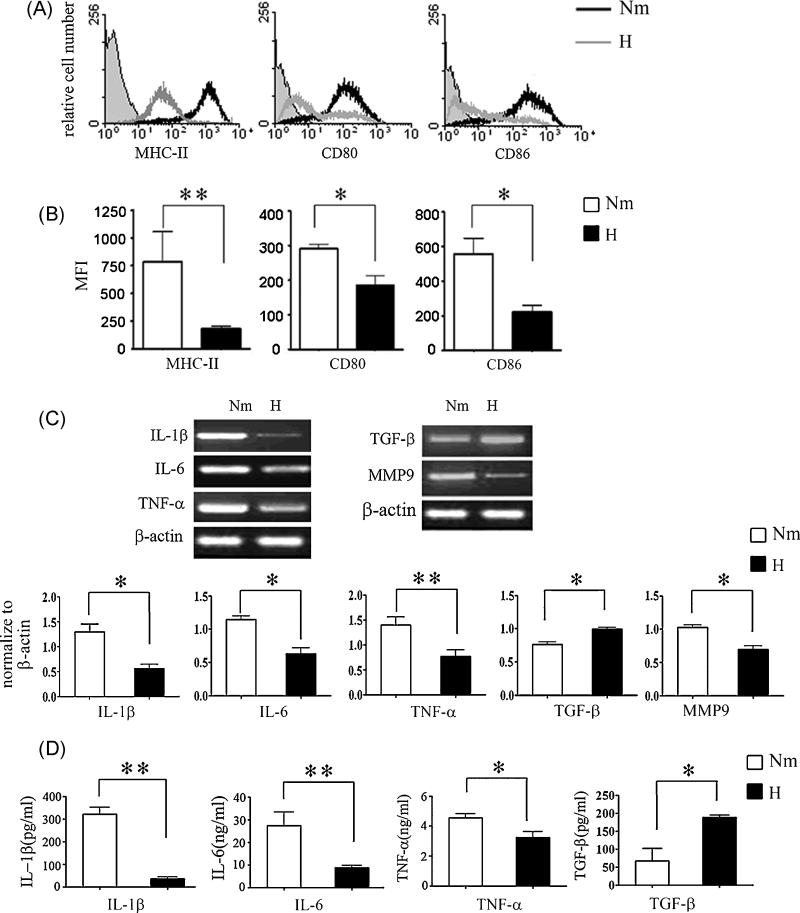
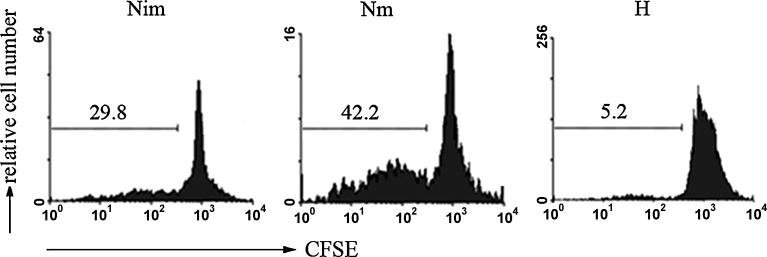
To investigate the effect of hypoxia on DC maturation, immature DCs were generated from murine BM progenitor cells with IL-4 and GM-CSF and stimulated by LPS for maturation under hypoxic or normoxic conditions, respectively. The expression of MHC-II, CD80 and CD86 on DCs was examined with flow cytometry. As shown in Fig. 1, DCs under hypoxic culture condition showed significantly decreased expression of MHC-II, CD80 and CD86 in response to LPS stimulation compared with normoxia-differentiated DCs (Fig.1A and B), suggesting that hypoxia-differentiated DCs maintain an immature or semi-mature phenotype. To investigate the effects of hypoxia on DC functions, we measured cytokine production of DCs by semi-quantitative RT-PCR and ELISA. We observed that hypoxia-differentiated DCs exhibited decrease in proinflammatory cytokines IL-1β, IL-6 and TNF-α, but increase in regulatory cytokine TGF-β in response to LPS treatment compared with normoxia-differentiated DCs (Fig. 1 C and D). We also noted that hypoxia-differentiated DCs expressed lower levels of MMP9 (Fig. 1C), a critical molecule for DC migration, as compared to normoxia-differentiated DCs, which was consistent with previous reports from hmDCs (Zhao et al., 2005; Qu et al., 2005; Zhao et al., 2008). We further examined T cell proliferation mediated by the different subsets of DCs using MLR assay. As shown in Fig. 2, hypoxia-differentiated DCs had poor ability to promote T cell proliferation compared with normoxia-differentiated DCs. Thus, hypoxia inhibited functional maturation of DCs.
Fig. 1. Phenotype and cytokine secretion of DCs generated under hypoxic or normoxic conditions.
(A) Murine BM-DCs were generated under normoxic or hypoxic conditions. Expressions of MHC-II, CD80, and CD86 on DCs were analyzed. Isotype controls, the indicated surface markers on LPS-treated normoxia-differentiated DCs (Nm), and LPS-treated hypoxia-differentiated DCs (H) are presented as filled, black, and grey lines in the histograms, respectively. Results are representative of four independent experiments. (B) The MFI of MHC-II, CD80, and CD86 expression mentioned in (A). Means ± S.D. is shown, *, P<0.05; **, P<0.01. (C) mRNA Levels for IL-1β, IL-6, TNF-α, TGF-β, and MMP9 of DCs generated as described in (A) were determined by RT-PCR, and normalized to β-actin. Data are representative of three independent experiments. Means ± S.D. is shown, *, P<0.05; **, P<0.01. (D) DCs were generated as mentioned in (A), supernatants were measured by ELISA for the secretion of IL-1β, IL-6, and TNF-α. Each sample was performed in triplicate wells. The data represent four independent experiments. Means ± S.D. is shown, *, P<0.05; **, P<0.01.
Fig. 2. Proliferation of CD4+ T cells induced by normoxia or hypoxia-differentiated DCs.
Normoxia-differentiated immature DCs (Nim), LPS-treated normoxia-differentiated (Nm) and hypoxia-differentiated (H) DCs were generated from C57BL/6 mice as described previously and treated with mitomycin C, then co-cultured respectively with CFSE-labeled CD4+ T cells (1×106/well) from the spleen of BALB/c mice at a ratio of 1:10 in the 24-well culture plates for 5 days. The ability for hypoxic or normoxic DCs to stimulate the proliferation of allogeneic CD4+ T cells were determined by flow cytometry. The percentage of dividing cells was shown in each histogram, data are representative of three independent experiments.
3.2. Reoxygenation of hypoxia-differentiated DCs completely restores their mature phenotype
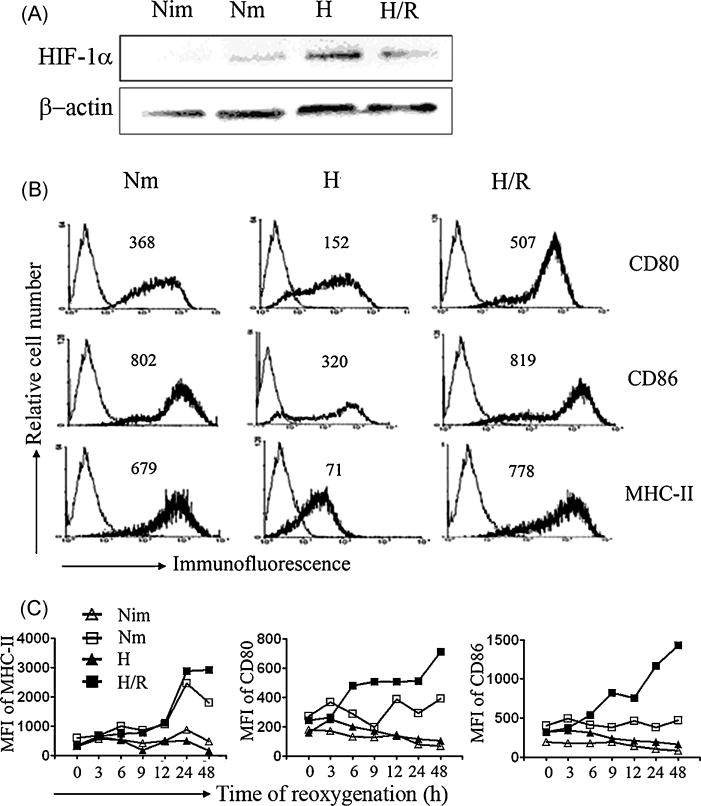
Although the pathogenesis of IRI is complex and not fully understood, a critical factor involved is the rapid reoxygenation in local microenvironment caused by the return of blood flow. Since our finding indicated that hypoxia inhibited the maturation of DCs, we then asked if the rapid change of oxygen tension could affect the phenotype and function of those hypoxia-differenriated DCs. For this purpose, hypoxic DCs were treated with LPS for 18h in hypoxia, then transferred into normoxic conditions (reoxygenation) for additional 6h, and the expression of HIF-1α and the changes of phenotypic molecules were examined by Western blot and flow cytometry analysis. The high levels of HIF-1α protein in hypoxic DCs reduced to almost undetectable levels as those in normoxic DCs after 6h of reoxygenation (Fig. 3A). Conversely, reoxygenation markedly upregulated the expression of CD80, CD86 and MHC-II on hypoxic DCs, which was even higher than that on LPS-treated normoxic DCs (Fig. 3B). Strikingly, even when LPS stimulated hypoxia-differentiated DCs were washed extensively to remove LPS and then re-cultured under hypoxic or normoxic conditions, the levels of CD80, CD86 and MHC-II expression were significantly restored and peaked at 24 to 48 h (Fig. 3C). These results indicate that LPS-treated hypoxia-differentiated DCs possess fully or even more potential to become mature DCs when sufficient oxygen is available.
Fig. 3. Effects of reoxygenation on phenotype change of hypoxic DCs.
(A) Normoxia-differentiated DCs with or without LPS treatment for 24 h were indicated as Nm and Nim, respectively. For reoxygenated culture, hypoxia-differentiated DCs were treated with LPS for 18 h then divided into two groups: one group was transferred into normoxia for additional 6 h, described as re-oxygenated dendritic cells (H/R); the other was continued to be kept in the condition of hypoxia for additional 6 h, named as hypoxic DCs (H). The expression of HIF-1α protein was determined by Western blot, each sample was performed at least three times. (B) Expression of MHC-II, CD80, and CD86 on DCs mentioned in (A) were analyzed by flow cytometry. Representative data of three experiments was shown, grey lines show the isotype controls, and black lines show the expression of the indicated molecules, MFI is presented as inserts. (C) Hypoxia-differentiated DCs were treated with LPS for 18 h, cells were harvested and washed twice sufficiently, then cultured in the 24-well culture plates (2×105/well) under hypoxic (H) or normoxic conditions (H/R) for different periods of time; the parallel experiments were performed on normoxia-differentiated DCs in the presence or absence of LPS (Nm or Nim). Expression of MHC-II, CD80, CD86 on different DCs were analyzed by flow cytometry, dynamics of MFI are representative of two independent experiments.
3.3. Reoxygenated DCs induced Th1 and Th17 cell differentiation with a decreased generation of CD4+CD25+Foxp3+ Tregs
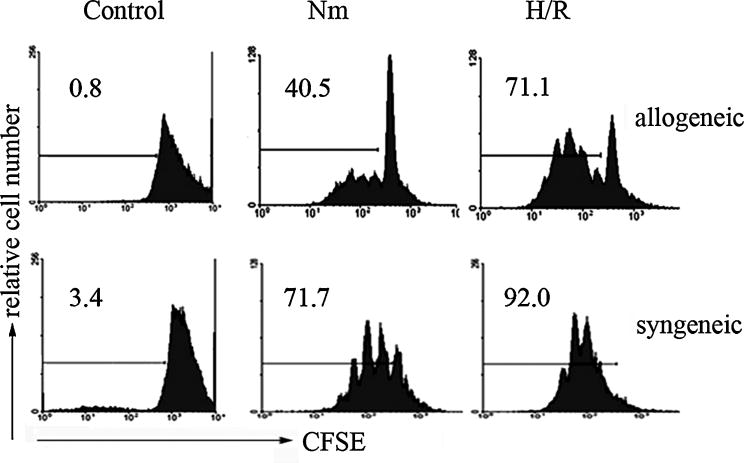
Although CD4+ effector T cells were reported to be involved in IRI (Huang et al., 2007; Marques et al., 2006), it is unknown whether reoxygenated DCs affected CD4+ T cell activation and differentiation. Since reoxygenation promoted the phenotype maturation of hypoxia-differentiated DCs, we hypothesized that these matured DCs influenced CD4+ T cell differentiation and function. To test this hypothesis, we analyzed the effects of reoxygenated DCs on the proliferation of allogeneic or syngeneic CD4+ T cells. In the proliferation assay, mitomycin-treated DCs from C57BL/6H-2b mice were co-cultured with CD4+ T cells from BALB/cH-2d (allogeneic) or C57BL/6 H-2b mice plus stimulation of CD3-specific antibody (syngeneic), respectively. DCs reoxygenated for 6 h stimulated vigorous CD4+ T cell proliferation, which was even stronger than normoxic mature DCs (for allogeneic CD4+ T cells, P<0.01; for syngeneic CD4+ T cells, P<0.05) (Fig. 4). Thus, reoxygenated DCs enhance CD4+ T cell proliferation.
Fig. 4. Proliferation of CD4+ T cells stimulated by reoxygenated DCs.
DCs from C57BL/6 mice (Nm, H/R) were generated as described in Fig. 3A, then treated with mitomycin C and co-cultured with CFSE-labeled CD4+ T cells from C57BL/6 (syngeneic) or BALB/c (allogeneic) mice under normoxic conditions for 3 or 5 days, 0.5μg/ml of anti-CD3 mAb was supplemented in the syngeneic cell proliferation assay. Proliferation of CD4+ T cells was determined by flow cytometry. Data are representative of three independent experiments.
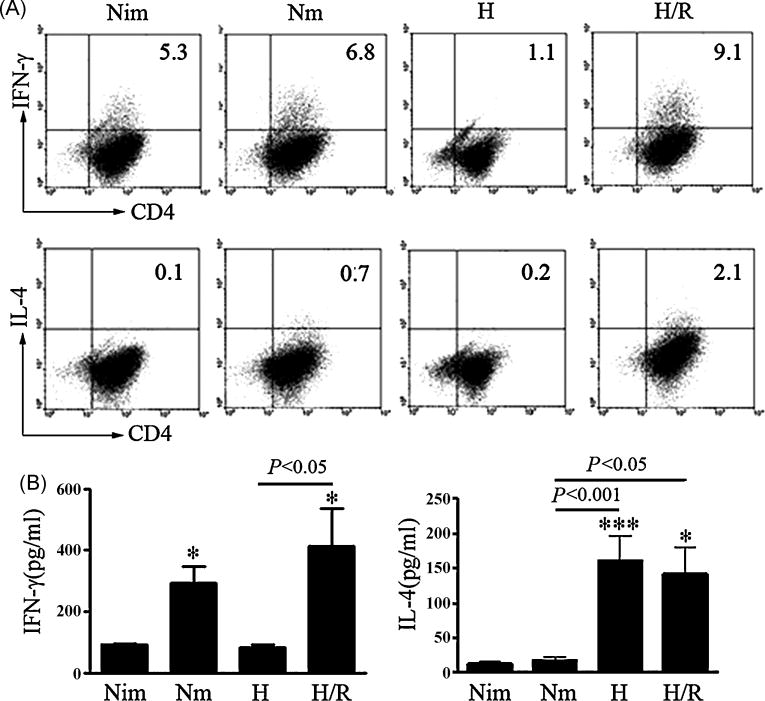
We then investigated the CD4+ T cell differentiation driven by reoxygenated DCs in the MLR culture systems. As shown in Fig. 5A, reoxygenated DCs induced significantly higher number of CD4+ IFN-γ+ T (Th1) cells than DCs differentiated under hypoxic or normoxic conditions did. The levels of IFN-γ in the supernatants of MLR systems was also higher in reoxygenated DCs than those in immature DCs (P<0.05) and normoxia-differentiated mature DCs (Fig. 5B). CD4+ IL-4+ T (Th2) cells induced by reoxygenated DCs were also elevated (Fig. 5A and B). These data indicate that reoxygenated DCs induce Th1 and Th2 cell differentiation.
Fig. 5. Differentiation of IFN-γ- and IL-4-producing cells in the coculture with DCs in response to reoxygenation.
(A) DCs from C57BL/6 mice generated under normoxic (Nim, Nm), hypoxic (H) and reoxygenated (H/R) conditions were treated with mitomycin C and co-cultured with allogeneic CD4+ T cells from BALB/c mice at a ratio of 1:10 in the 24-well culture plates for 5 days (MLR). Expression of IFN-γ and IL-4 in MLR systems were measured by intracellular staining. Representative data of three independent experiments were shown. (B) Supernatants from MLR cultures as described in (A) were collected at day 5 and examined for IFN-γ and IL-4 secretion. Results were from four independent experiments, Means ± S.D. was shown, *, *** represent P<0.05, P<0.001 versus Nim respectively, using one way ANOVA.
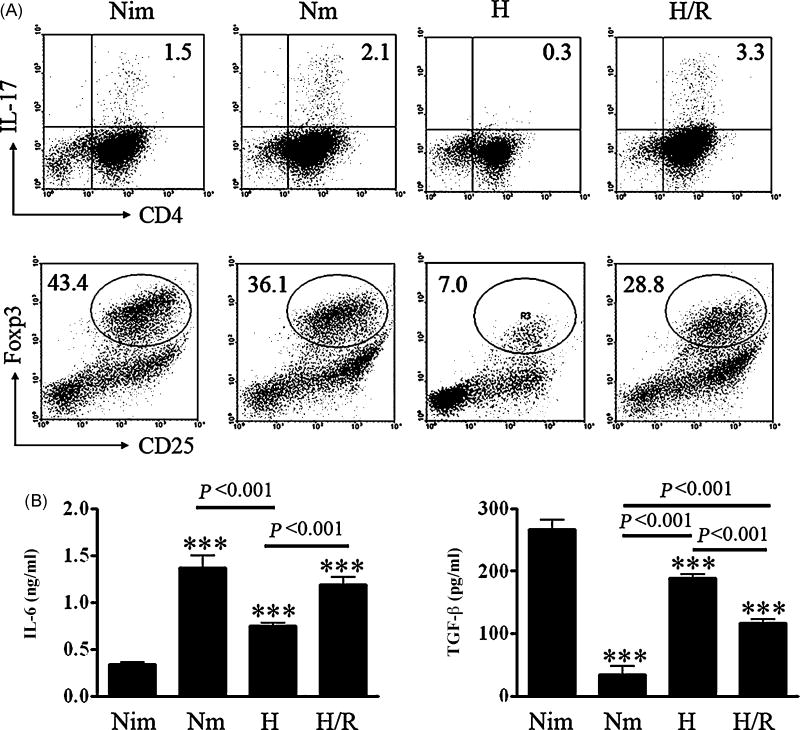
Considering the critical roles of Th17 cells and Tregs in inflammation and immune tolerance, we examined the effects of reoxygenated DCs on the differentiation of Th17 and Tregs. Strikingly, reoxygenated DCs induced much more IL-17+CD4+ T (Th17) cells from naïve CD4+ T cells, but substantially lower levels of CD4+CD25+Foxp3+ Tregs compared with normoxia-differentiated mature DCs (Fig. 6A). Since IL-6 abrogates TGF-β-induced Foxp3+ Tregs but together with TGF-β drives the differentiation of Th17 cells (Bettelli et al., 2006; Mangan et al., 2006; Qin et al., 2009; Veldhoen et al., 2006), we then analyzed the cytokine profile of reoxygenated DCs. We found that hypoxic DCs exposed to oxygen for 24 h produced significantly higher levels of IL-6 and slightly lower levels of TGF-β compared with that in hypoxia-differentiated DCs (Fig. 6B), suggesting the elevated IL-6 secretion by reoxygenated DCs along with significant amounts of TGF-β abrogates Treg generation but drives CD4+ T cells toward Th17 cells. Taken together, these results suggest that reoxygenated DCs have strong capacity to drive immune response toward a proinflammatory direction.
Fig. 6. Differentiation of IL-17-producing cells and Foxp3+ T cells in the coculture with DCs in response to reoxygenation.
(A) MLR was executed as described in Fig. 5A, 5 days later, cells were collected for IL-17A and Foxp3 staining according to the manufacturer’s instructions. Representative data of three independent experiments were shown. (B) Different DCs were prepared and cultured as described in Fig. 3C, supernatants from the DCs cultures were collected at 24 h and assayed for IL-6 and TGF-β secretion by ELISA. Results were from three independent experiments, Means ±S.D. was shown, *** represents P <0.001 versus Nim using one way ANOVA.
4. Discussion
In this study, we demonstrated that hypoxic microenvironment suppresses the maturation and function of murine BM-DCs, while reoxygenation of hypoxia-differentiated DCs can reverse this suppression, promote the maturation and function of DCs and drive the Th1 and Th17-mediated immune responses, at the expense of Treg conversion. Our results suggest that strong Th1 and Th17 immune responses induced by reoxygenated DCs may contribute to IRI.
Although several studies have addressed the impact of hypoxic microenvironmet on phenotypes and functions of DCs, the results from various labs are inconsistent. A few of reports (Zhao et al., 2005; Qu et al., 2005; Yang et al., 2006) have demonstrated that hypoxia suppresses the migration of human monocyte-derived DCs in vitro by downregulating MMP-9 expression and augmenting the protease inhibitor TIMP1. In accord with these data, we also observed that hypoxia downregulated MMP9 mRNA levels in murine BM-DCs. Furthermore, Mancino et al. demonstrate that hypoxia inhibit the upregulation of CCR7 and DC homing to draining lymph node in vivo (Mancino et al., 2008). However, the gene expression profile of hypoxic DCs done by Ricciardi et al. showed that increased CCR2 and CXCR4 endow hypoxic DCs with high sensitivity to chemoattractants, suggesting there is no intrinsic defect in the migration of hypoxic DC (Bosco et al., 2008; Ricciardi et al., 2008). About the effect of hypoxia on maturation of DCs, two researches show that hypoxia have no effect on maturation of human monocyte-derived DCs and their ability to trigger T cell response (Elia et al., 2008; Zhao et al., 2005), whereas another study reports that hypoxia promotes the maturation and function of murine BM-DCs (Jantsch et al., 2008). In contrast, Mancino A. et al. demonstrates that hypoxia inhibited the expression of maturation markers (CD1a, CD40, CD80, CD83, CD86, and MHC class II molecules) of human monocyte-derived DCs accompanied by their reduced ability to prime T-cell functions (Mancino et al., 2008). In agreement with this, our results indicate that LPS fails to induce the maturation of DCs in hypoxic microenvironment as evidenced by decreased expression of MHC-II and co-stimulatory molecules (CD80 and CD86), and inhibition of their capability to stimulate the proliferation of CD4+ T cells. In addition, our results show that hypoxia also reduces the secretion of proinflammatory cytokines (IL-1β, IL-6, TNF-α) and upregulate anti-inflammatory cytokine (TGF-β), however, which is not in line with previous studies on human monocyte-derived DCs, which show that hypoxia can upregulate proinflammatory cytokine activity (Elia et al., 2008; Mancino et al., 2008; Ricciardi et al., 2008). Together, all previous studies and our results demonstrate that hypoxic microenvironment affects the differentiation, maturation and function of DCs, but precise impacts of hypoxia on DCs are still controversial. The reasons for these discrepancies may include following aspects: (1) the different hypoxia treatments on DC generation, maturation. In our present and a few previous researches, both induction and maturation of DCs were performed in hypoxia (Zhao et al., 2005; Qu et al., 2005), while in some other studies, induction of DCs was in normoxia but their maturation was in hypoxia (Elia et al., 2008; Mancino et al., 2008), which may cause the disagreement of results; (2) the difference of species (human vs murine) and individuals, may be another factor for the distinct characteristics between human monocyte-derived DCs and murine BM-DCs on precursors, surface markers, signal pathways, even some functions; (3) cell reoxygenation also can not be ignored since cell reoxygenation can strongly affect various biologic function including DCs maturation and their ability to stimulate adaptive immunity as mentioned by Mancino A et al. and our provided data (Fig. 3, 4). In our early research, we also found that LPS-treated hypoxic DCs promoted but not inhibited CD4+ T cell proliferation although their maturation phenotypes were significantly suppressed by hypoxia. However, further investigation provided evidence that this discrepancy resulted from reoxygenation of hypoxia-differentiated DCs. To avoid the effect of reoxygenation on phenotype and function of DCs in our study, the medium for hypoxic culture was balanced in hypoxic condition at least for 24 h, the time for replacing medium was kept as short as possible, and cells were fixed before staining and DCs were treated by mitomycin C in T cell proliferation assay as described by Mancino A. et al (Mancino et al., 2008). In addition, to indicate the status of hypoxia, the expression of HIF-1α, a major regulator for cell adaptation to hypoxia, was detected.
Taken together, our data support that hypoxia suppresses the maturation and functions of DCs. Recently, the growing evidence that demonstrate adenosine function as endogenous inhibitor of immune response, may help to partially explain our results. Excess accumulation of extracellular adenosine has long be implicated in the adaption to hypoxia, which is achieved in ischemic tissue and enough to active A2BR on immune cells to exert anti-inflammatory effects, thereby preventing tissue injury (Hasko et al., 2009). Zhao P et al. indicates that hypoxia altered the expression of adenosine receptors on matured DCs toward the predominant expression of A2B adenosine receptor (A2BR) and the activation of A2BR suppresses the production of MMP-9. Other studies (Ben Addi et al., 2008; Wilson et al., 2009) report that the activation of A2BR reduced the expression of MHC class II and CD86, and inhibits LPS-induced production of proinflammatory cytokines such as IL-12, TNF-α, IL-6 but promote secretion of anti-inflammatory cytokines such as TGF-β, IL-10 (Hasko et al., 2009; Wilson et al., 2009). Recently, our early results show that hypoxia-differentiated DCs express high levels of A2BR, and both exogenous adenosine and adenosine receptor nonselective agonist NECA can downregulate the expression of surface molecules. (data not shown). Hypoxia may inhibit the proinflammatory cytokines by activation of adenosine receptor A2BR. We thus propose that hypoxia-differentiated DCs maintain an immature status, preferably induce an immune tolerance rather than Th immune responses. Therein, hypoxia-differentiated DCs themselves contribute little if anything, to the inflammation of ischemia phase in IRI. Accordingly, the low level of co-stimulatory molecules and the reduced proinflammatory cytokines in hypoxia-differentiated DCs may be also involved in this self-protective process. However, exact mechanism need to be investigated in future.
We provided evidence that reoxygenation could reverse the suppression of DCs mediated by hypoxia. The expression of MHC class II molecule and co-stimulatory molecules on hypoxic DCs increased markedly within a few hours and reached the peak by 48 h after reoxygenation, consequently acquired strong ability to stimulate Th-mediated immune responses. Consistent to these observations, recent studies have shown an accumulation and activation of DCs in IRI (Kim et al., 2005; Tsung et al., 2005). In addition, as noted in Mancino et al.’s study, strong effects of rexygenation on various biological functions were also observed in hypoxic human DCs (Mancino et al., 2008). We propose a novel viewpoint that reoxygenation, characterized by sudden rapid oxygen delivery, acts as a potential accelerant to promote the maturation of hypoxic DCs, which reverses the immunosuppressive status and triggers stronger adaptive Th-mediated inflammatory responses. It could be envisioned that the reperfusion of ischemic areas, in particular readmission of oxygen, may exaggerate or cause additional injuries not present at the end of ischemia (Cao et al., 2006; Leonard et al., 2006).
Our observation that reoxygenated DCs induce strong Th1 and Th17 cell differentiation is novel and significant. A few studies have suggested that CD4+ rather than CD8+ T cell subsets are involved in various IRI models. It has been reported that CD4+ T cells regulated neutrophil recruitment and tissue injury in hepatic IRI via an IL-17-dependent mechanism (Caldwell et al., 2005). In the present study, we determined that re-exposure to oxygen led hypoxia differentiated DCs to drive a strong Th17 and Th1 cell responses. Our data added DCs-mediated Th17 cell responses into the list of the cellular mechanisms responsible for the pathogenesis of IRI in animal models (Bonventre and Zuk, 2004; Huang et al., 2007). Moreover, the decrease in CD4+CD25+Foxp3+ Tregs generation by reoxygenated DCs further strengthens the idea that these DCs play a critical function in IRI. Recent studies demonstrate that TGF-β is required for both Th17 and Treg cell differentiation. TGF-β in the context of T cell receptor stimulation leads to the conversion of Foxp3+ Tregs (Chen et al., 2003; Luo et al., 2007; Saas et al., 2007), whereas TGF-β plus IL-6 result in Th17 cells (Bettelli et al., 2006; Mangan et al., 2006; Qin et al., 2009; Veldhoen et al., 2006). To investigate the mechanism that reoxygenated DCs induced much more Th17 cells, we showed that reoxygenated DCs produced higher levels of IL-6 and relatively lower levels of TGF-β compared to immature DCs, providing a molecular basis to understand predominant Th17 cell differentiation and reduction of Foxp3+ Tregs.
Taken together, the hypoxic microenvironment suppresses the maturation and function of murine DCs. Re-exposure of the hypoxia-differentiated DCs to saturated oxygen however results in complete recovery of their mature phenotype and function, and has strong ability to drive immune response toward a proinflammatory direction.
Acknowledgments
This study was supported by research funding from the National Natural Science Foundation of China (30628015, 30700729), National “973” program of China (2006CB503803) and Natural Science Foundation of Shandong Province, China (Q2007C02) and the Intramural Research Program of the NIH, NIDCR, USA. We thank Dr. Youhai Chen, University of Pennsylvania, USA and Xun Qu, Institute of Basic Medical Sciences, Qilu Hospital, Shandong University, China for critical discussions on this manuscript.
Abbreviations
- DC
dendritic cells
- Tregs
regulatory T cells
- IL
interleukin
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- IFN
interferon
- LPS
lipopolysaccharides
- HIF-1α
hypoxia induced factor-1 alpha
- MLR
mixed lymphocyte reaction
- IRI
ischemia-reperfusion injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand RJ, Gribar SC, Li J, Kohler JW, Branca MF, Dubowski T, Sodhi CP, Hackam DJ. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1alpha-dependent manner. J Leukoc Biol. 2007;82:1257–1265. doi: 10.1189/jlb.0307195. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Addi A, Lefort A, Hua X, Libert F, Communi D, Ledent C, Macours P, Tilley SL, Boeynaems JM, Robaye B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur J Immunol. 2008;38:1610–1620. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M, Varesio L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G969–976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- Cao L, Li Y, Cheng F, Li S, Long D. Hypoxia/reoxygenation up-regulated the expression of death receptor 5 and enhanced apoptosis in human hepatocyte line. Transplant Proc. 2006;38:2207–2209. doi: 10.1016/j.transproceed.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005;68:1096–1108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- Elia AR, Cappello P, Puppo M, Fraone T, Vanni C, Eva A, Musso T, Novelli F, Varesio L, Giovarelli M. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84:1472–1482. doi: 10.1189/jlb.0208082. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–270. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, Eckardt KU, Steinkasserer A, Hensel M, Willam C. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutala VK, Khan M, Angelos MG, Kuppusamy P. Role of oxygen in postischemic myocardial injury. Antioxid Redox Signal. 2007;9:1193–1206. doi: 10.1089/ars.2007.1636. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O’Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. Faseb J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Loi P, Paulart F, Pajak B, Nagy N, Salmon I, Moser M, Goldman M, Flamand V. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant Proc. 2004;36:1275–1279. doi: 10.1016/j.transproceed.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, Steinman RM, Suthanthiran M. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells. Proc. Natl Acad Sci U S A. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Marques VP, Goncalves GM, Feitoza CQ, Cenedeze MA, Fernandes Bertocchi AP, Damiao MJ, Pinheiro HS, Antunes Teixeira VP, dos Reis MA, Pacheco-Silva A, Saraiva Camara NO. Influence of TH1/TH2 switched immune response on renal ischemia-reperfusion injury. Nephron Exp Nephrol. 2006;104:e48–56. doi: 10.1159/000093676. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, Benveniste EN, Cong Y. TGF-{beta} Promotes Th17 Cell Development through Inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi A, Elia AR, Cappello P, Puppo M, Vanni C, Fardin P, Eva A, Munroe D, Wu X, Giovarelli M, Varesio L. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol Cancer Res. 2008;6:175–185. doi: 10.1158/1541-7786.MCR-07-0391. [DOI] [PubMed] [Google Scholar]
- Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saas P, Bonnefoy F, Kury-Paulin S, Kleinclauss F, Perruche S. Mediators involved in the immunomodulatory effects of apoptotic cells. Transplantation. 2007;84:S31–34. doi: 10.1097/01.tp.0000269113.59857.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–1312. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108:1189–1197. doi: 10.1182/blood-2006-01-007187. [DOI] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Wang JY. Hypoxia/reoxygenation induces cell injury via different mechanisms in cultured rat cortical neurons and glial cells. Neurosci Lett. 2002;322:187–191. doi: 10.1016/s0304-3940(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Wenli Zhao SD. Hypoxia suppresses the production of matrix metalloproteinases and the migration of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35:3468–3477. doi: 10.1002/eji.200526262. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, Linden J, Ernst PB. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009;182:4616–4623. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Kroemer A, Gao W, Ishii N, Demirci G, Li XC. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181:3193–3201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- Qu X, Yang MX. hypoxia iinhibits the migration capacity of human monocyte-derived dendritic cells. Immunol Cell Biol. 2005;83:668–673. doi: 10.1111/j.1440-1711.2005.01383.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal. 2008;10:755–768. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- Yang MX, Qu X, Kong BH, Lam QL, Shao QQ, Deng BP, Ko KH, Lu L. Membrane type 1-matrix metalloproteinase is involved in the migration of human monocyte-derived dendritic cells. Immunol Cell Biol. 2006;84:557–562. doi: 10.1111/j.1440-1711.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu S, Song H, Song B, Zhang Y, Qu X. Hypoxia suppresses the production of MMP-9 by human monocyte-derived dendritic cells and requires activation of adenosine receptor A2b via cAMP/PKA signaling pathway. Mol Immunol. 2008;45:2187–2195. doi: 10.1016/j.molimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Zhou T, Sun GZ, Zhang MJ, Chen JL, Zhang DQ, Hu QS, Chen YY, Chen N. Role of adhesion molecules and dendritic cells in rat hepatic/renal ischemia-reperfusion injury and anti-adhesive intervention with anti-P-selectin lectin-EGF domain monoclonal antibody. World J Gastroenterol. 2005;11:1005–1010. doi: 10.3748/wjg.v11.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]