Abstract
Aim
Some cardiac phenomena demonstrate temporal variability. We evaluated temporal variability in out-of-hospital cardiac arrest (OHCA) frequency and outcome.
Methods
Prospective cohort study (the Resuscitation Outcomes Consortium) of all OHCA of presumed cardiac cause who were treated by emergency medical services within 9 US and Canadian sites between 12/1/2005 and 02/28/2007. In each site, Emergency Medical System records were collected and analyzed. Outcomes were individually verified by trained data abstractors.
Results
There were 9,667 included patients. Median age was 68 (IQR 24) years, 66.7 % were male and 8.3% survived to hospital discharge. The frequency of cardiac arrest varied significantly across time blocks (p<0.001). Compared to the 0001–0600 hourly time block, the odds ratios and 95% CIs for the occurrence of OHCA were 2.02 (1.90, 2.15) in the 0601–1200 block, 2.01 (1.89, 2.15) in the 1201–1800 block, and 1.73 (1.62, 1.85) in the 1801–2400 block. The frequency of all OHCA varied significantly by day of week (p=0.03) and month of year (p<0.001) with the highest frequencies on Saturday and during December. Survival to hospital discharge was lowest when the OHCA occurred during the 0001–0600 time block (7.3%) and highest during the 1201–1800 time block (9.6%). Survival was highest for OHCAs occurring on Mondays (10.0%) and lowest for those on Wednesdays (6.8%) (p=0.02).
Conclusion
There is temporal variability in OHCA frequency and outcome. Underlying patient, EMS system and environmental factors need to be explored to offer further insight into these observed patterns.
INTRODUCTION
Out-of-hospital cardiac arrest (OHCA) is a significant public health problem, with the North American incidence of EMS-treated cardiac arrest estimated to be 52.1 per 100,000 people(1). Understanding the chronobiology of OHCA is important to clarify the immediate precipitants of sudden cardiac death, develop preventative strategies, and to optimize resource planning for the prehospital and in hospital response to cardiac arrest.
Several potential triggers for cardiac arrest have demonstrated circadian variability. Examples include physical activity, psychological stress, increases in heart rate, blood pressure and sympathetic tone, reduced fibrinolytic activity, and increased platelet aggregation(2). Epidemiological reports support underlying chronobiological mechanisms for cardiac arrest, demonstrating circadian (3–10), circaseptan(8, 9, 11) and circannual(8, 9) variability in the frequency of OHCA. However, few studies have used a geographically-diverse, population-based prospective registry of EMS-attended cardiac arrest as the basis for reporting. Furthermore, few studies have investigated whether survival from OHCA varies according to time of day, day of week or month of year. Patient characteristics, arrest circumstances, and treatment could vary according to time period and could in turn influence the likelihood of survival(12).
The objective of this investigation was to evaluate temporal variability in the frequency and outcome of OHCA in a large, heterogeneous and unselected population.
METHODS
Ethics approval for this study was obtained from 74 US Institutional Review Boards and 34 Canadian Research Ethics Boards as well as 26 EMS Services Institutional Review Boards.
Study design, population, and setting
The Resuscitation Outcomes Consortium (ROC) Epistry – Cardiac Arrest is a large, population-based prospective registry of OHCA episodes collected from 11 regional centers across the U.S. and Canada. The methods for Epistry, including a complete description of the included population and EMS services have been described in detail (13–15). Each ROC site had to ensure capture of all eligible OHCA cases. Examples of case identification strategies included use of paramedic collection of ambulance call reports for cardiac arrests at the time of the event, regular hand sorting through paper EMS charts, and electronic queries of EMS records by a variety of data fields.
We conducted a prospective cohort study of consecutive adult subjects (≥20 years old) suffering non-traumatic OHCA of presumed cardiac cause who were treated by EMS or fire service responders from December 1, 2005 to February 28, 2007 from 9 of the ROC sites. Two sites were excluded from the current investigation because of insufficient ascertainment of OHCA episodes. We excluded untreated OHCA as this often represents persons who have been dead for a relatively long time, in whom an accurate estimate of time of death is not possible. Criteria for EMS non-treatment by those responders legally authorized to make such a judgement, were clear signs of death such as the presence of decomposition or rigour mortis or because of a “do not attempt resuscitation” directive authorized by a physician, or for compelling reasons that included extensive history of terminal illness or intractable disease, and/or a request from the patient’s family(13). We also excluded OHCA which occurred in nursing homes or other health care institutions because these patients were thought to represent a population distinct from the majority of OHCA cases. Episodes with incomplete data abstraction or missing witness status were excluded because of planned sub-group analyses. Our analysis of variability by hour of day included a planned sensitivity analysis including all EMS-attended OHCAs to assess the potential impact of these exclusions on our final conclusions.
Data collection and data definitions
The time and date of the call received at dispatch was used as a surrogate measure for the time of OHCA occurrence(13). We evaluated occurrence and outcome of OHCA by the time of day, day of week and month of year. Time of day was divided into four 6-hour time intervals (0001–0600, 0601–1200, 1201–1800, 1801–2400) chosen to maintain comparability with previous reports(8–10). For analyses evaluating temporal variability in outcomes from OHCA, the outcome was survival to hospital discharge.
Statistical Analysis
We used hot deck imputation methods to create an imputed dataset for missing data(16). We combined both original and imputed data from December 1, 2005 to November 30, 2006 with complete, original data from December 1, 2006 to February 28, 2007 to form our study dataset. Ten percent of the cases were imputed.
A pre-specified subgroup analysis evaluated survival to hospital discharge by hour of day among patients with an initial cardiac arrest rhythm that was shockable (ventricular fibrillation, pulseless ventricular tachycardia or AED shock) because this is a subgroup least influenced by factors outside the control of EMS which tends to have a higher baseline survival rate. Post hoc subgroup analyses evaluated 1) whether occurrence of OHCA varied by day of the week when stratified by age (>60 versus < 60) since prior studies suggest a Monday excess for employed persons(9); 2) whether occurrence of OHCA varied by season, because environmental factors such as cold temperature and higher snowfall have been hypothesized to affect OHCA risk; and 3) whether survival varied according to weekday versus weekend (Friday 5 pm to Monday 8 am) since circumstances and health resources can vary between these two time periods.
Patient and event characteristics were examined by time block; significance across time blocks was analyzed with χ2 square goodness of fit tests. Mean age and mean EMS times (total out-of-hospital, response, on-scene, transport, time to CPR, time to Shock) were analyzed with a linear ANOVA model. Hourly, day of week, and month of year frequencies were analyzed graphically and with χ2 square goodness of fit tests. We used multiple logistic regression models to examine the association between survival to discharge and the time block, day of week and month of year when the cardiac arrest occurred. The multivariable models were adjusted for sex, age (20–39, 40–60, 61–75, >75), initial rhythm (VF/VT, PEA, Asystole, AED-No Shock, Cannot Determine), witness status, bystander resuscitation (resuscitation attempt, CPR attempt, AED applied and no shock, shock given), EMS response time (0–5, 5–10, >10)and ROC site. Confidence intervals for the time unit coefficients were calculated using methods presented by Rubin(16). A p-value of less than 0.05 was considered statistically significant. All analyses were performed with S-Plus 6.2 (Insightful Corporation, Seattle, Washington).
RESULTS
Description of the cohort
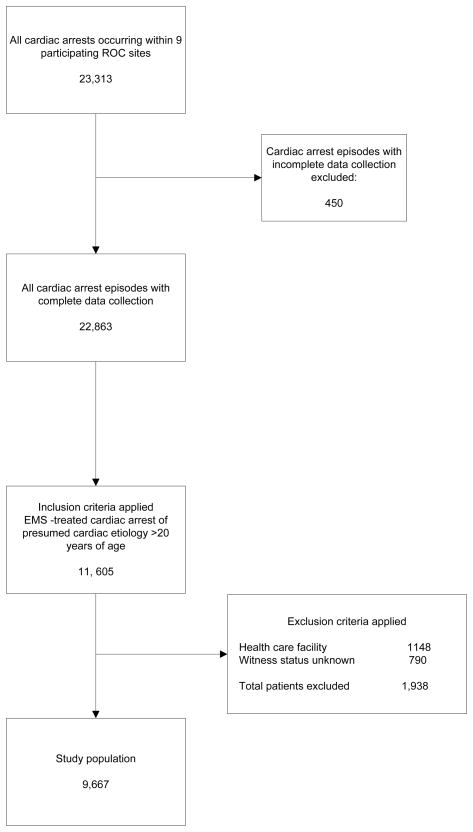
There were 23,313 OHCAs occurring within the 9 participating ROC sites from December 1, 2005 to February 28 2007. Four hundred fifty cases (1.9%) were excluded for missing data. Only 57.5% of these patients were treated by EMS. Nine thousand six hundred sixty-seven patients met criteria for inclusion in the study (Figure 1). Overall survival to hospital discharge was 8.3%.
Figure 1.
The study cohort.
Demographics, cardiac arrest characteristics and EMS response characteristics by time block are shown in Table 1. The initial cardiac arrest rhythm, location of arrest, bystander CPR and AED use differed significantly across time blocks. Emergency medical services median response intervals including total out-of-hospital time, on scene time, time to CPR and time to shock were the longest during the 0001–0600 time block, but this did difference not reach statistical significance.
Table 1.
Patient and event details for 9,667 patients with out-of-hospital cardiac arrest.
| Total (n = 9667) | 0001–0600 (n = 1428) | 0601–1200 (n = 2887) | 1201–1800 (n = 2877) | 1801–2400 (n = 2475) | p-value* | |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 68 (24) | 65 (26) | 69 (24) | 70 (23) | 68 (24) | 0.70 |
| Male, n (%) | 6448 (66.7) | 959 (67.1) | 1935 (67.0) | 1946 (67.6) | 1609 (65.0) | 0.21 |
| Initial Rhythm | <0.001 | |||||
| VF/VT, n (%) | 2682 (27.7) | 346 (24.2) | 764 (26.5) | 861 (29.9) | 711 (28.7) | |
| PEA, n (%) | 1892 (19.6) | 282 (19.7) | 539 (18.7) | 552 (19.2) | 519 (21.0) | |
| Asystole, n (%) | 3601 (37.3) | 592 (41.4) | 1135 (39.3) | 1004 (34.9) | 871 (35.2) | |
| AED no-shock, n (%) | 1002 (10.4) | 148 (10.5) | 303 (10.5) | 285 (9.9) | 265 (10.7) | |
| Unknown, n (%) | 490 (5.1) | 60 (4.2) | 146 (5.1) | 175 (6.1) | 110 (4.4) | |
| Location of Arrest | <0.001 | |||||
| Public, n (%) | 1752 (18.1) | 94 (6.6) | 512 (17.7) | 741 (25.8) | 406 (16.4) | |
| Private, n (%) | 7910 (81.8) | 1335 (93.4) | 2376 (82.3) | 2132 (74.1) | 2067 (83.5) | |
| Unknown, n (%) | 5 (0.0) | 0 | 0 | 4 (0.1) | 1 (0.0) | |
| Witness Status | <0.001 | |||||
| Witnessed, n (%) | 5213 (53.9) | 814 (57.0) | 1406 (48.7) | 1627 (56.6) | 1365 (55.2) | |
| Unwitnessed, n (%) | 4454 (46.1) | 614 (43.0) | 1481 (51.3) | 1250 (43.4) | 1109 (55.8) | |
| Bystander Resuscitation | <0.001 | |||||
| CPR Attempted, n (%) | 2801 (29.0) | 384 (26.9) | 773 (26.8) | 927 (32.2) | 718 (29.0) | |
| AED Shock, n (%) | 151 (1.6) | 4 (0.3) | 37 (1.3) | 85 (3.0) | 25 (1.0) | |
| AED Applied no shock, n (%) | 69 (0.1) | 10 (0.7) | 20 (0.7) | 21 (0.7) | 18 (0.7) | |
| Median (IQR) EMS Time Intervals in minutes | ||||||
| Total out of hospital | 38 (17) | 41 (18) | 38 (18) | 37 (17) | 39 (16.4) | 0.46 |
| Response | 6 (3) | 6 (3) | 5 (3) | 5 (3) | 6 (3) | 0.87 |
| On Scene | 24 (14) | 27 (14.5) | 24 (14) | 23 (14) | 24 (14) | 0.61 |
| Transport | 6 (6) | 7 (5.4) | 6 (6) | 6 (6) | 6 (6) | 0.99 |
| Time to CPR | 8 (4) | 10 (5) | 8 (5) | 8 (5) | 8 (4) | 0.75 |
| Time to Shock† | 11 (11) | 13 (11.9) | 11 (11) | 11 (10) | 11 (11) | 0.56 |
Abbreviations: VF/VT = ventricular fibrillation or ventricular tachycardia, PEA = pulseless electrical activity, AED no shock = automated external defibrillator no shock advised, CPR=cardiopulmonary resuscitation, EMS = Emergency medical services, AED shock = automated external defibrillator shock advised, IQR = interquartile range, Total out of hospital = time interval from call received at dispatch to prehospital death or emergency department arrival. Response = time interval from time call received at dispatch to first vehicle at scene. On Scene = time interval from first vehicle arrival on scene to prehospital death or transport from scene. Transport = time interval from transport from scene to emergency department arrival. Time to CPR = time interval from call received at dispatch to onset of EMS cardiopulmonary resuscitation. Time to shock = time from call received at dispatch to the first EMS defibrillation attempt for those with a rhythm of VF/VT.
Time intervals were compared across time blocks with an ANOVA model with a covariance matrix that accounts for multiple imputation. All other variables were compared across time blocks with a Chi-squared goodness-of-fit test.
Among those with ventricular fibrillation or pulseless ventricular tachycardia.
Out-of-hospital cardiac arrest frequency
Hour of Day
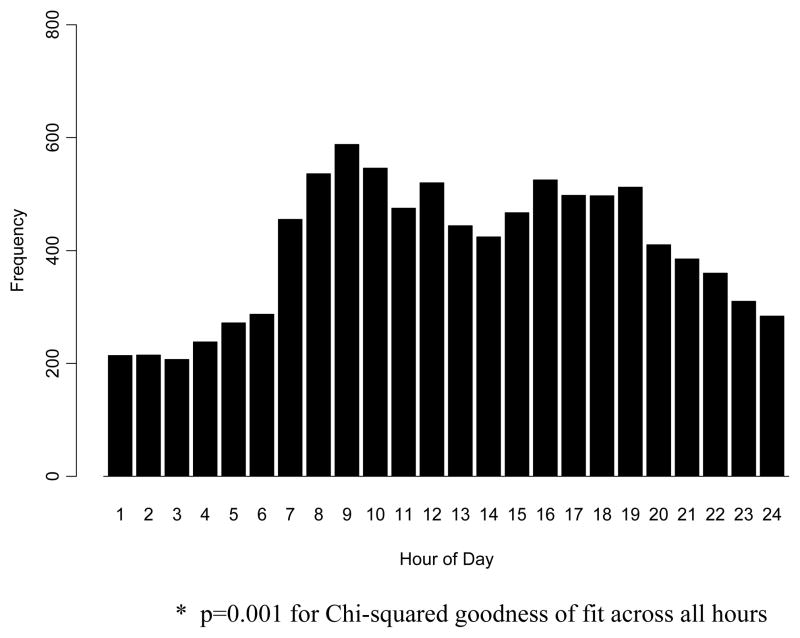
The distribution of OHCA by hour of day can be seen in Figure 2. Cardiac arrest frequency demonstrates a distribution with a peak between 0800 and 1000 and a secondary peak between 1600 and 1900. The frequency of cardiac arrest varied significantly across time blocks (p<0.001). Compared to the 0001–0600 time block, the odds ratios and 95% CIs for the occurrence of OHCA in each time block were 2.02 (1.90, 2.15) for 0601–1200 hrs, 2.01 (1.89,2.15) 1201–1800 hrs, and 1.73 (1.62, 1.85) for 1801–2400 hrs. This general pattern of daytime excess was observed regardless of gender, initial rhythm, whether or not the arrest was witnessed, and location of arrest (data not shown). A sensitivity analyses that included all 23,313 EMS-attended (treated and untreated) OHCAs produced a very similar distribution with a morning peak and a night time nadir.
Figure 2.
The distribution of 9,667 out-of-hospital cardiac arrests by hour of day.
Day of Week
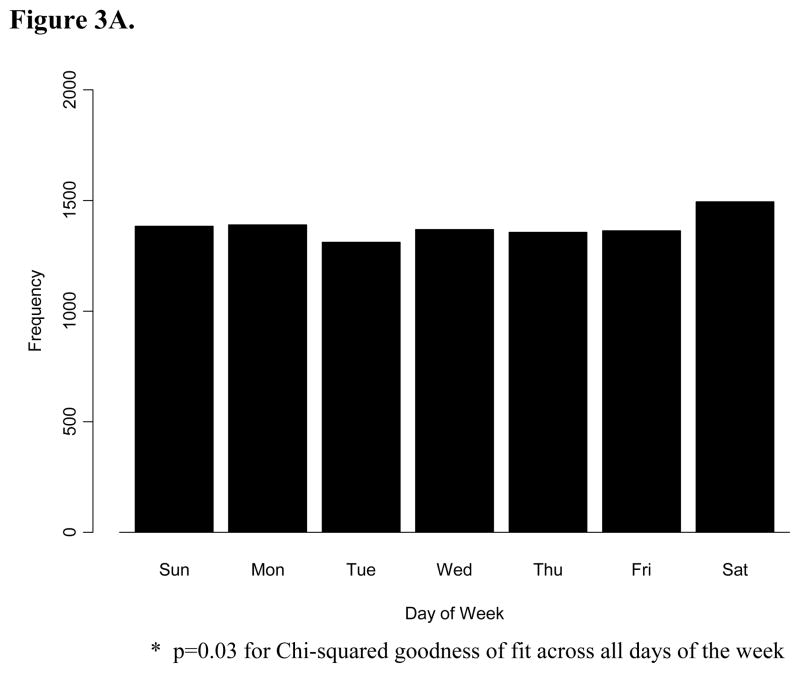
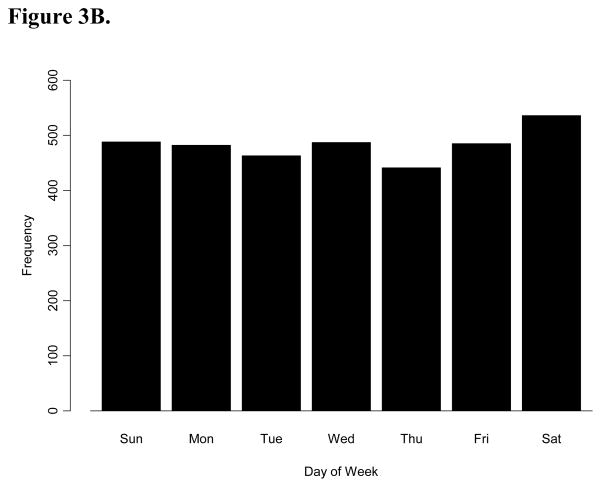
The frequency of all OHCA varied significantly by day of week (p=0.03), with the highest frequency on Saturday (n=1494) and the lowest frequency on Tuesday (n=1311) (Figure 3A). In a post-hoc analysis, we tested the hypothesis that there may be a Monday or weekday excess of cardiac arrests among people who are part of the workforce. We examined the subgroup less than 60 years of age (Figure 3B), under the assumption that this age group was more likely to be pre-retirement. We found no evidence for significant variation across day of week among this sub-group (p=0.11).
Figure 3.
A. The frequency distribution of 9,667 out-of-hospital cardiac arrests by day of week. B. The frequency distribution of 3, 382 patients less than 60 years old with out-of-hospital cardiac arrests by day of week.
Month of Year
Average cardiac arrest frequency varied significantly by month, with the maximum number occurring in December (p<0.001). The mean number of cardiac arrests ranged from 570 in February to 742 in December. To aid in the interpretation of these findings, we looked for a seasonal trend by grouping months into Winter (December to February), Spring (March to May), Summer (June to August) and Fall (September to November). There was no evidence for frequency variability across seasons (p=0.06).
Survival to hospital discharge
Hour of Day
Survival to hospital discharge was lowest when the OHCA occurred during the 0001–0600 time block and highest during the 1201–1800 time block (Table 2). In the unadjusted analysis, patients in the 1201–1800 group had 36% higher odds of survival to hospital discharge than those in the 0001–0600 time block. After multivariate adjustment, there was no statistically significant association between time block of the cardiac arrest and survival to hospital discharge. In the sub-group analysis of those with VT/VF/AED shock advised, there was no evidence for an association between time block and survival to hospital discharge (data not shown).
Table 2.
Survival to hospital discharge by time block
| Time block | Number of Episodes | Number (%)Survived | Odds Ratios for Survival with 95% CIs | |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| 0001–0600 | 1428 | 105 (7.3%) | - | - |
| 0601–1200 | 2887 | 219 (7.6%) | 1.04 (0.80, 1.35) | 0.99 (0.74, 1.34) |
| 1201–1800 | 2877 | 277 (9.7%) | 1.36 (1.05, 1.75) | 1.08 (0.84, 1.54) |
| 1801–2400 | 2475 | 202 (8.2%) | 1.13 (0.87, 1.46) | 0.96 (0.71, 1.29) |
Unadjusted and adjusted odds ratios for survival to hospital discharge among 9,667 patients with out-of-hospital cardiac arrest. Unadjusted odds ratios were derived from a simple logistic regression model exploring the relationship between time block and survival to hospital discharge. Adjusted odds ratios were derived from a multiple logistic regression model adjusting for sex, age, initial rhythm, witness status, bystander CPR, bystander automated external defibrillator use, EMS response time and ROC site.
Abbreviations: CI = confidence interval
Day of Week
Survival varied significantly by day of week (p=0.02). The highest rate of survival to hospital discharge was observed when the OHCA occurred on a Monday (10.0%) and the lowest rate was observed when the initial arrest happened on a Wednesday (6.8%). Table 3 shows odds ratios for survival to hospital discharge using Wednesday as the reference. Survival did not differ on weekends compared to weekdays (unadjusted OR 1.00 (95% CI 0.85, 1.18), adjusted OR 0.99 (95% CI 0.82, 1.19)).
Table 3.
Survival to hospital discharge by day of week.
| Number of episodes | Number (%)survived | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Sunday | 1384 | 109 (7.9) | 1.17 (0.87, 1.56) | 1.04 (0.75, 1.44) |
| Monday | 1390 | 140 (10.0) | 1.53 (1.16, 2.03) | 1.52 (1.10, 2.11) |
| Tuesday | 1311 | 103 (7.9) | 1.16 (0.87, 1.56) | 0.92 (0.66, 1.29) |
| Wednesday | 1369 | 94 (6.8) | -- | -- |
| Thursday | 1356 | 115 (8.5) | 1.27 (0.95, 1.70) | 1.18 (0.85, 1.63) |
| Friday | 1363 | 100 (7.3) | 1.08 (0.81, 1.46) | 0.90 (0.63, 1.26) |
| Saturday | 1494 | 144 (9.6) | 1.46 (1.09, 1.97) | 1.35 (0.96, 1.90) |
Unadjusted and adjusted odds ratios for survival to hospital discharge by day of week among 9,667 patients with out-of-hospital cardiac arrest. Unadjusted odds ratios were derived from a simple logistic regression model exploring the relationship between day of week and survival to hospital discharge. Adjusted odds ratios were derived from a multiple logistic regression model adjusting for sex, age, initial rhythm, witness status, bystander CPR, bystander automated external defibrillator use, EMS response time and ROC site.
Month of Year
We observed the highest rate of survival to discharge among patients who suffered their cardiac arrest in July (12.2%) and the lowest rate of survival for those who had their cardiac arrest in September (6.7%), however, variation across months was not statistically significant (p=0.06).
DISCUSSION
In this large, population-based, multisite study using data from the Resuscitation Outcomes Consortium Epistry – Cardiac Arrest, we observed temporal variability in OHCA occurrence and outcome. These temporal observations provide a framework to advance our understanding of the triggers of OHCA. Such a framework can potentially improve resuscitation care through EMS resource planning and cardiac arrest research which incorporates this phenomenon as a potential variable affecting the risk for cardiac arrest and the probability of a favourable outcome.
Limitations
When interpreting the results, one must consider the limitations and strengths of this investigation in the context of prior research on the topic. We were unable to relate the timing of cardiac arrest to actual behaviours such as wake time, physical activities or medication compliance. Temporal variability studies of OCHA are limited by their ability to accurately determine the precise time of OHCA occurrence. Studies using death certificates and hospital records to determine time for out-of-hospital cardiac arrest(3–5) are likely to be inaccurate given the delayed nature of the record-keeping inherent in these circumstances. Studies which use other more accurate measures, such as cardiac tracings from implantable cardiac defibrillators(17), have limited generalizability because of selection bias in the type of patients that tend to have these devices implanted. Using the time of 911 call received at dispatch, although not as vulnerable to these problems, is also an imperfect measure and is a limitation of this study. It is likely that the probability of a cardiac arrest being witnessed or discovered shortly after onset is variable over the course of the day due to human sleep/wake behaviour. We were unable to account for any temporal variability in the use of post resuscitation therapies, such as therapeutic hypothermia, which may have contributed to the observed temporal variability in survival to hospital discharge. The current study investigates out-of-hospital cardiac arrest. Our findings may not be pertinent to in-hospital cardiac arrests. We undertook multiple comparisons and findings should be interpreted with caution given the possibility of type I error.
Out-of-hospital cardiac arrest frequency
We observed a daytime excess and overnight nadir of OHCA occurrence which is generally consistent with previous studies (3–10). For example, a recent population-based registry from multiple EMS services in Sweden reported a very similar frequency distribution among 10,868 OHCAs with a peak occurrence between 0900 and 1000(8). Data from a single EMS service in Berlin(9) and a multi-center study from Belgium(18) reveal a very similar distribution of OHCA with peaks in the morning and late afternoon. Our database has allowed us to confirm the observed circadian rhythms amongst important subgroups defined by key variables such as initial rhythm and witness status.
Data on circadian rhythms in the frequency of acute myocardial infarction, unstable angina, stroke, and hypothesized physiologic triggers for sudden cardiac arrest also support circadian variation in OHCA(10, 19–21). Autopsy data demonstrating that 90% of patients with sudden cardiac death have recently ruptured coronary atherosclerotic plaques suggests that the majority of sudden cardiac deaths are secondary to acute myocardial ischemia and related arrhythmias(19). The incidence of non-fatal myocardial infarction, which has a more reliable time stamp of pain onset, is approximately 40% higher during the 0600–1200 time block compared to all other times of the day(10, 20). Several other sudden cardiovascular events including ischemic stroke(21), pulmonary embolism(22) and ventricular arrhythmias(17, 23, 24) exhibit a very similar daytime excess and morning peak in frequency.
Cardiac arrest frequency varied significantly by day of week with the maximum number of OHCAs occurring on Saturdays. Perhaps behaviour changes related to weekends such as increased levels of strenuous physical activity, food intake, and altered medication compliance might be responsible for the observed increase in frequency. In contrast to earlier studies, we found no evidence for a higher frequency of cardiac arrest on Monday, even among a younger sub-group of patients more likely to be working. The underlying hypothesis that psychological stress associated with return to work on Mondays may trigger sudden cardiac arrest(8, 9, 11) is not supported by our data.
Although we found evidence for variability across months, our data does not support the hypothesis of seasonal variability in the frequency of OHCA, which is in contrast to previous research from other geographic locations(8, 9). The discrepancy may be due to the previous results being spurious, geographically specific, related to local climate-related behaviour, or different underlying pathophysiology of OHCA in different geographical locations. The sites included in this analysis are climactically varied. This heterogeneity may have impaired our ability to detect a true seasonal or environmental effect. In a post-hoc fashion, we did graphically explore the data (data not presented) with sites grouped by environmental conditions, using a dichotomous, arbitrary annual snowfall amount to sort the sites, but despite this, we failed to observe any significant trend in frequency by month of the year.
Survival to hospital discharge
The odds of surviving an out-of-hospital cardiac arrest are lowest when the call to 911 occurs between 0001 and 0600 hours. Odds for survival to discharge during the afternoon time block of 1201–1800 are 36% higher than during the 0001–0600 time block. Patients who suffered cardiac arrest during the 1201–1800 time block had the advantage of more public location cardiac arrests resulting in more bystander CPR and a higher rate of AED use. Patients with OHCA during the 0001–0600 time block were at a disadvantage for survival because the event likely occurred during sleep and recognized after some delay. Arrests over night happened more frequently in private residences where AEDs are uncommon (Table 1). This variability in circumstances and cardiac arrest characteristics may explain our finding that the difference in survival is no longer apparent when we adjust for covariates such as initial rhythm, witness status, bystander CPR, bystander AED use and EMS response time. The observed difference in survival is likely due to temporal variability among some or all of these factors rather than another unrecognized source of variability. In a recent study of in-hospital cardiac arrest(12), reduced survival in patients with night time arrest persisted after adjustment for multiple covariates including the covariates included in the current multivariable model evaluating OHCA outcome. The difference in adjusted results between the prehospital and in-hospital cohorts may be due to different patient characteristics, EMS versus hospital staffing and response, and unmeasured factors such as CPR performance, rescuer mindset or effort, which may be contributing to variability in the hospital cohort.
We have also observed variability in survival to hospital discharge after OHCA by day of week, with the highest observed survival associated with Monday OHCAs. One interpretation is that the greater survival on Monday is a chance finding. Alternatively, a wide variety of factors may vary by day of the week and could affect the predisposition, pathophysiology and ultimately the survival of OHCA. These may include 1) EMS staffing and performance factors (e.g. CPR quality), 2) patient factors such as behaviour, physiologic environment and the underlying cause of OHCA and, 3) hospital factors such as staffing patterns and access to interventions or expertise.
CONCLUSION
In the Resuscitation Outcomes Consortium Epistry database, there is evidence for circadian variability in OHCA frequency with a daytime excess of out-of-hospital cardiac arrests an overnight nadir. Although OHCA frequency also varies significantly by day of week and month of year with most occurring on Saturdays and during December, there are no clear trends with respect to weekday versus weekend or across seasons of the year. Survival to hospital discharge varies by hour of day and is least likely for those who suffer a cardiac arrest between the hours of 0001 and 0600. This can be explained by patient and event characteristics known to predict survival, which also vary by hour of day and are least favourable for survival over night during this time block. Cardiac arrest survival is highest on Mondays, however there is no evidence for higher survival during the week as compared with weekends. Underlying patient, EMS system and environmental mechanisms need to be explored to offer further insight into these observed patterns. The growing body of evidence demonstrating significant temporal variation in OHCA, especially by hour of day, should support future chronobiological intervention studies, including timed therapies, to target periods of highest risk.
Acknowledgments
The Resuscitation Outcome Consortium (ROC) is supported by a series of cooperative agreements to 10 regional clinical centers and one data Coordinating Center (5U01 HL077863, HL077881, HL077871 HL077872, HL077866, HL077908, HL077867, HL077885, HL077885, HL077863) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, The National American Heart Association, and the Heart and Stroke Foundation of Canada.
The authors would like to thank all of the front line emergency services providers who have contributed to this study through primary data collection and a commitment to supporting resuscitation research.
Footnotes
CONFLICT OF INTEREST STATEMENT
Steven Brooks, Robert Schmicker, Thomas Rea, Laurie Morrison, Ritu Sahni, Denise Griffiths, Scott Emerson and George Sopko have no disclosures relevant to this manuscript.
Tom P. Aufderheide is a paid consultant for Medtronics Inc. and JoLife. He sits on the Board of Directors for Take Heart America. He has received honoraria from EMS Today.
Daniel P. Davis has received an unrestricted research grant from Zoll Medical for an investigator-initiated study. He is a paid consultant to Cardinal Health.
Gena Sears is a shareholder for Medtronic Corporation.
Paul Dorian has received a research grant from Zoll Medical Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Thomas E, Callaway CW. Regional Variation in Out-of-Hospital Cardiac Arrest Incidence and Outcome. JAMA. 2008;300(12):1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–43. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 3.Willich SN, Levy D, Rocco M, Tofler G, Stone P, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham heart study population. Am J Card. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 4.Willich SN, Goldberg R, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Card. 1992;70:65–8. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 5.Willich SN. Epidemiologic studies demonstrating increased morning incidence of sudden cardiac death. Am J Card. 1990;66(16):G15–G7. doi: 10.1016/0002-9149(90)90387-g. [DOI] [PubMed] [Google Scholar]
- 6.Thakur R, Hoffmann RG, Olson DW, Joshi R, Tresch DD, Aufderheide TP, et al. Circadian variation in sudden cardiac death: Effects of age, sex, and initial cardiac rhythm. Ann Emerg Med. 1996;27(1):29–34. doi: 10.1016/s0196-0644(96)70292-5. [DOI] [PubMed] [Google Scholar]
- 7.Peters R, Muller JE, Goldstein S, Byington R, Friedman L for the BHAT Study Group. Propanolol and the morning increase in the frequency of sudden cardiac death (BHAT Study) Am J Card. 1989;63:1518–20. doi: 10.1016/0002-9149(89)90019-2. [DOI] [PubMed] [Google Scholar]
- 8.Herlitz J, Eek M, Holmberg M, Holmberg S. Diurnal, weekly and seasonal rhythm of out of hospital cardiac arrest in Sweden. Resuscitation. 2002 Aug;54(2):133–8. doi: 10.1016/s0300-9572(02)00097-7. [DOI] [PubMed] [Google Scholar]
- 9.Arntz HR, Willich SN, Schreiber C, Bruggemann T, Stern R, Schultheib H. Diurnal, weekly and seasonal variation of sudden death. Eur Heart J. 2000;21:315–29. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Card. 1997;79(11):1512. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 11.Willich SN, Lowel H, Lewis M, Hormann A, Arntz HR, Keil U. Weekly variation of acute myocardial infarction. Increased Monday risk in the working population. Circulation. 1994;90(1):87–93. doi: 10.1161/01.cir.90.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299(7):785–92. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 13.Morrison LJ, Nichol G, Rea TD, Christenson J, Callaway CW, Stephens S, et al. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry--Cardiac Arrest. Resuscitation. 2008;78:161–9. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newgard CD, Sears GK, Rea TD, Davis DP, Pirrallo RG, Callaway CW, et al. The Resuscitation Outcomes Consortium Epistry-Trauma: Design, development, and implementation of a North American Epidemiologic Prehospital Trauma Registry. Resuscitation. 2008;78:170–8. doi: 10.1016/j.resuscitation.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis D, Garberson L, Andrusiek D, Hostler D, Daya M, Pirrallo R, et al. A descriptive analysis of emergency medical service systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11(4):369–82. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 17.Kozak M, Krivan L, Semrad B. Circadian variations in the occurrence of ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2003 Mar;26(3):731–5. doi: 10.1046/j.1460-9592.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 18.Martens P, Calle P, Van den Poel B, Lewi P the Belgian Cardiopulmonary Cerebral Resuscitation Study Group. Further prospective evidence of a circadian variation in the frequency of call for sudden cardiac death. Intensive Care Med. 1995;21:45–9. doi: 10.1007/BF02425153. [DOI] [PubMed] [Google Scholar]
- 19.Willich SN, Maclure M, Mittleman M, Arntz HR, Muller JE. Sudden cardiac death. Support for a role of triggering in causation. Circulation. 1993;87(5):1442–50. doi: 10.1161/01.cir.87.5.1442. [DOI] [PubMed] [Google Scholar]
- 20.Leiza JR, de Llano JM, Messa JB, Lopez CA, Fernandez JA the Ariam Study Group. New insights into the circadian rhythm of acute myocardial infarction in subgroups. Chronobiol Int. 2007;24(1):129–41. doi: 10.1080/07420520601140027. [DOI] [PubMed] [Google Scholar]
- 21.Elliott WJ. Circadian Variation in the Timing of Stroke Onset: A Meta-analysis. Stroke. 1998;29(5):992–6. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 22.Colantonio D, Casale R, Abruzzo B, Lorenzetti G, Pasqualetti P. Circadian distribution in fatal pulmonary thromboembolism. Am J Card. 1989;64(5):403–4. doi: 10.1016/0002-9149(89)90548-1. [DOI] [PubMed] [Google Scholar]
- 23.Lampert R, Rosenfeld L, Batsford W, Lee F, McPherson C. Circadian variation of sustained ventricular tachycardia in patients with coronary artery disease and implantable cardioverter- defibrillators. Circulation. 1994;90(1):241–7. doi: 10.1161/01.cir.90.1.241. [DOI] [PubMed] [Google Scholar]
- 24.Tofler GH, Gebara OCE, Mittleman MA, Taylor P, Siegel W, Venditti FJ, Jr, et al. Morning Peak in Ventricular Tachyarrhythmias Detected by Time of Implantable Cardioverter/Defibrillator Therapy. Circulation. 1995;92(5):1203–8. doi: 10.1161/01.cir.92.5.1203. [DOI] [PubMed] [Google Scholar]