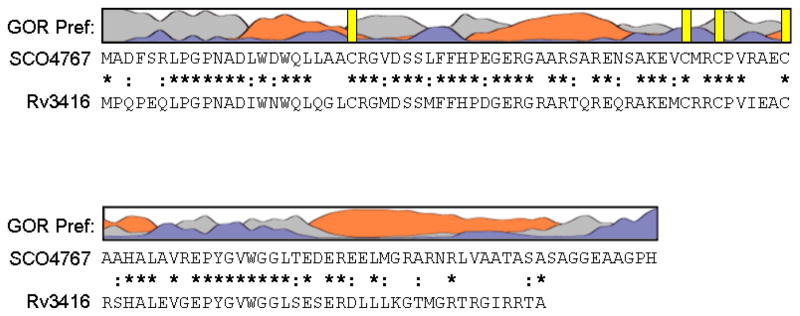
Figure 1. Sequence analysis of WhiD (SCO4767).
The secondary structure of WhiD was predicted using GOR IV (73). The resulting prediction indicated approximately 42% α-helix, 55% coil and 3% beta strand. Preference for α-helix is indicated by orange, β-sheet by blue and coil by grey. Vertical yellow bars indicate the position of cysteine residues. Sequence alignment of WhiD (SCO4767) and WhiB3 (Rv3416) from M. tuberculosis H37Rv is shown. Conserved residues are indicated by a star, conservatively substituted residues are indicated by a colon.