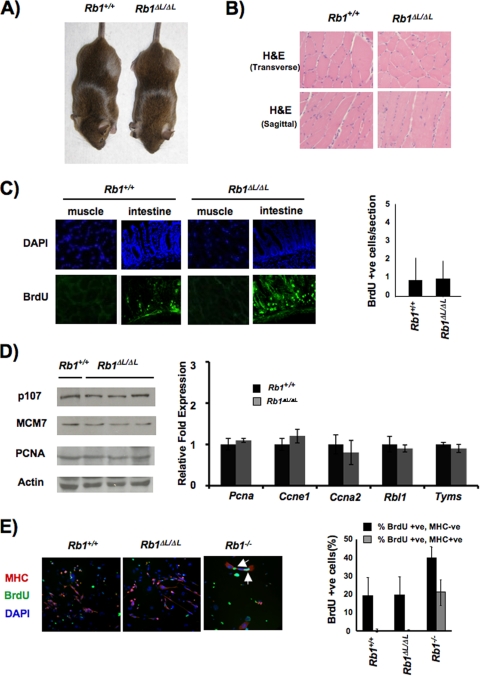
FIG. 2.
Normal cell cycle exit and differentiation of muscle. (A) Rb1ΔL/ΔL mutant animals are viable and appear indistinguishable from wild-type littermates. (B) Anterior tibialis muscle tissue from 8- to 10-week-old animals was stained with H&E to examine the gross morphology of the wild type and Rb1ΔL/ΔL mutants. Transverse and sagittal sections are shown. (C) Cell proliferation in muscle was examined by BrdU staining, the number of BrdU-positive cells per microscopic field was quantified, and the average is displayed in the graph. As a control for detection of BrdU in mature muscle fibers, we also stained cryosections of intestinal epithelia prepared from the same mice. DAPI, 4′,6-diamidino-2-phenylindole. (D) mRNA and protein were extracted from the muscle of 6-week-old wild-type and Rb1ΔL/ΔL mutant mice. Western blot assays show the expression of known E2F target genes, and the graph to the right displays the relative abundance of the specified transcripts. Message levels of acidic ribosomal phosphoprotein P0 (Rplp0) and actin protein levels were used as controls. (E) The indicated genotypes of MEFs were infected with MyoD-expressing retroviruses and induced to differentiate into myocytes under low-serum conditions. Cells were then restimulated with 15% fetal bovine serum and pulse-labeled with BrdU (24 h) to detect DNA synthesis. Myocytes were identified by MHC staining (red), DNA synthesis was detected by BrdU staining (green), and DNA was counterstained with DAPI (blue). The percentage of myocytes (MHC positive) and surrounding fibroblasts (MHC negative) that incorporated BrdU in response to serum is shown in the graph to the right. The error bars in all of the graphs indicate 1 standard deviation from the mean of at least three replicates.