Abstract
There are few data about the epidemiology of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) among children in Israel. This study was intended to identify risk factors for CA-MRSA colonization in healthy infants, to characterize the molecular features of colonizing organisms, and to determine whether they are responsible for health care-associated (HA) infections. Nasal cultures and demographic details were collected from a cohort of healthy infants at 5 visits between the ages of 2 and 12 months. Clinical characteristics of pediatric MRSA bloodstream infections (2001 to 2006) and wound cultures collected over 6 months were also studied. Clonal structure was evaluated by multilocus sequence typing. Isolates were studied for the staphylococcal cassette chromosome mec (SCCmec) type and for the presence of Panton-Valentine leukocidin (PVL) genes. MRSA was cultured at least once from 45 of 659 infants (346 Jewish and 313 Bedouin infants). Forty of 45 (89%) isolates were from Bedouin infants. Twenty-nine of 45 (64.4%) belonged to a new clonal complex, designated CC913, that carries SCCmec IV but not the PVL genes. CC913 was also isolated from 9/14 blood cultures and 7/8 wounds. All CC913 infections occurred in Bedouin children, and all but two were HA. In conclusion, Bedouin origin was the main risk factor for carriage of CA-MRSA. CC913 was dominant both in healthy carriers and as a cause of pediatric HA-MRSA bloodstream infections.
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has become a worldwide problem, although its prevalence differs considerably among countries. Consistently high prevalence is found in the United States, South America, Japan, and southern Europe, whereas prevalence is low in Scandinavia, the Netherlands, and Switzerland (12, 22, 24, 25, 44).
Clusters and outbreaks of CA-MRSA infections have been reported in “closed populations,” such as Native Americans (19), prison inmates (5), children attending child care centers (1), military recruits (50), and competitive sports participants (5). Moreover, CA-MRSA, originally found mainly in the community, has now been introduced into the hospital setting (22, 31, 38). At some hospitals, CA-MRSA strains are even displacing classical hospital-acquired (HA) strains of MRSA (45).
There are few reports of CA-MRSA in Israel. A recent study reported a low prevalence of MRSA carriage (0.11%) in children attending outpatient clinics in central Israel (36). One of the earliest CA-MRSA outbreaks reported in the literature occurred in 1997 among 20 patients in a facility for adults with developmental disabilities in southern Israel (4). Isolates from all the outbreaks had a single SmaI macrorestriction pattern, but other molecular characteristics were not sought.
The IsraPrev study is an ongoing investigation, initiated in August 2005, designed to evaluate the effects of different scheduling protocols of a 7-valent pneumococcal conjugate vaccine (PCV7) on the carriage of Streptococcus pneumoniae and S. aureus in healthy infants. During this study, a surprisingly high prevalence of MRSA carriage (up to 4.4% of all children) was noted, almost exclusively among Bedouin infants.
Our objectives were (i) to investigate the clonal structure and molecular characteristics of MRSA strains isolated from healthy infants in southern Israel, (ii) to determine whether CA-MRSA has penetrated the hospital environment and to characterize the clinical features of CA-MRSA infections, (iii) to explore the relationship between CA-MRSA strains in southern Israel and previously characterized clones (Israeli and global), and (iv) to identify risk factors for MRSA carriage in healthy infants in southern Israel.
MATERIALS AND METHODS
Setting.
In southern Israel (the Negev region), Jewish and Bedouin populations, differing in socioeconomic conditions and lifestyles, live side by side. However, both have access to the same medical services. The Jewish population is mainly urban, whereas the Bedouin population is in transition from a nomadic existence in the desert to a Western lifestyle (6). Contact between children of the two populations is rare. The Bedouin population is characterized by overcrowding, a lower education level, a larger family size, and a lower income than the Jewish population (6). In 2004, the crude birth rate was 55.3 versus 21.0 per 1,000 for the Bedouin and Jewish populations (3), respectively, and the mean family size (± standard deviation) was 8.2 ± 0.9 persons for the Bedouin population versus only 3.2 ± 0.1 persons for the Jewish population (7). The average monthly family income was 2-fold higher for the Jewish population (3). Hospitalization rates for respiratory and other infectious diseases were higher among Bedouin children than among Jewish children (26).
All children in the area are born in one hospital, where they also receive all emergency and inpatient services. More than 60% of children in the Negev region are medically insured by the largest health plan in Israel, the General Health Insurance Plan.
Patients (i) IsraPrev study.
MRSA carriage and molecular characterization of MRSA strains were determined for infants participating in the IsraPrev study, as described in the introduction. Healthy 2-month-old infants were enrolled and evaluated at 5 visits (at the ages of 2, 4, 6, 7, and 12 months). At each visit, demographic data (age, gender, number of children) and medical history (antibiotic use; previous illness, such as acute otitis media [AOM] or asthma) were gathered, and swabs of the anterior nares were collected for S. aureus isolation. The study protocol was approved by the ethics committee of the Soroka University Medical Center.
(ii) Clinical cases.
All cases of pediatric (<18 years) MRSA bloodstream infection (BSI) from 2001 to 2006 were included. All children who had MRSA isolated from wound cultures during a 6-month period in 2006 were included. Health care-associated MRSA cases were defined as cases where patients had either (i) an MRSA infection identified after 48 h of admission to a hospital, (ii) a history of hospitalization, surgery, dialysis, or residence in a long-term care facility within 1 year prior to the MRSA culture date, (iii) a permanent indwelling catheter or percutaneous medical device (e.g., a tracheostomy tube, gastrostomy tube, or Foley catheter) present at the time of culture, or (iv) a known positive culture for MRSA prior to the study period (28).
(iii) Outbreak strains.
Isolates of MRSA collected during an outbreak of skin infections that occurred in 1997 in an institution for adults with mental disabilities (4) were studied for their molecular characteristics.
Microbiological methods.
Antimicrobial susceptibility testing was performed by a disk diffusion assay. Resistance to methicillin in S. aureus was tested by a cefoxitin disk diffusion method and confirmed by an agglutination test for the production of PBP2a (Oxoid Ltd., Cambridge, United Kingdom). Screening for vancomycin-intermediate/resistant Staphylococcus aureus was performed by the agar screening plate method, according to CLSI recommendations (9).
(i) PFGE.
SmaI macrorestriction patterns were determined as previously described (17). Briefly, cell suspensions were mixed 1:1 with 1.2% agar (SeaKem Gold agarose; Lonza, Rockland, ME) and were poured into wells. Agar plugs were lysed in a shaker incubator, first with lysozyme (1 mg/ml) for 4 h in 37°C and then with proteinase K (1 mg/ml) for 4 h at 50°C. Plugs were washed six times in Tris-EDTA (TE) solution. Plugs were cut and digested for 2 h with 30 U of SmaI enzyme. The plugs were then inserted into pulsed-field gel electrophoresis (PFGE) gels (1% SeaKem Gold agarose), and the gels were run on a CHEF Mapper XA system (catalog no. 170-3672; Bio-Rad, Hercules, CA). The PFGE pulse ramp was 5 to 15 s for the first 10 h and then 15 to 60 s for the last 13 h. The voltage was 6 V/cm, and the angle was 120°. Gels stained with ethidium bromide were photographed with a ChemiDoc XRS camera (catalog no. 170-8070; Bio-Rad, Hercules, CA) and were analyzed with Fingerprinting II software, version 3.0 (Bio-Rad, Hercules, CA). Isolates were considered related if the macrorestriction patterns had ≤6 bands different, according to the criteria of Tenover et al. (43).
(ii) MLST.
Multilocus sequence typing (MLST) was performed on representative strains from each PFGE cluster and subcluster, according to a standardized protocol (15). MLST results were analyzed by eBURST software, version 3 (http://www.mlst.net).
(iii) SCCmec typing.
Staphylococcal cassette chromosome mec (SCCmec) typing was performed by multiplex PCR (49). PCR was carried out with a PCR Master Mix (Promega, Madison, WI) with a Biometra TGradient Thermo Cycler apparatus (Biomedizinische Analytik GmbH, Göttingen, Germany). Ambiguous results were also tested by single-target PCR for the ccr and the mec complexes (49).
(iv) Determination of the agr type and the presence of the PVL and arcA genes.
agr typing was performed by multiplex PCR (28). The presence of the lukS-PV and lukF-PV genes (27) and the arcA gene (13) was tested by PCR. These genes were sought because they are present in many CA-MRSA strains around the world (Panton-Valentine leukocidin [PVL] genes) (14, 27, 29, 33) or in the USA300 strain (arcA) (13), and thus, they may play a role in the virulence of CA-MRSA infections.
Data analysis and statistical methods.
Molecular characteristics were studied for all patient-unique MRSA isolates detected in the IsraPrev study, the clinical cases, and the outbreak isolates. Risk factors for MRSA carriage were studied only for infants who had attended all first 5 visits of the IsraPrev study. Variables included in the analysis were (i) demographic (age, gender, ethnicity, and type of residency for Bedouin children), (ii) socioeconomic (number of siblings, number of people in the child's room, smoking in the infant's home, breastfeeding), and (iii) health care related (antibiotic use since the last visit and previous hospitalization).
To detect differential risk factors for MRSA carriage, comparisons were made as follows: MRSA versus methicillin-susceptible S. aureus (MSSA) and S. aureus carriers (total) versus noncarriers. No distinction was made between transient and persistent carriers.
Continuous variables were assessed using the t test, and categorical variables were assessed with the χ2 or Fisher exact test, as appropriate. In multivariate analysis, performed for S. aureus carriers (total) versus noncarriers, risk factors found to be related to the dependent categorical variable at a P value of ≤0.1 were entered into logistic regression models.
Survival analyses were carried out using the Kaplan-Meier method. For all analyses, α was set at 0.05. All analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows, version 14.0; SPSS, Inc.).
RESULTS
A total of 659 infants (346 Jewish and 313 Bedouin infants) were recruited from September 2005 to June 2006. Of those, 553 infants participated in all of the first 5 visits and were thus included in the risk factor analysis. Of the 553 infants participating in the risk factor analysis, MRSA was isolated from nasal cultures of 32 infants (5.7%) in at least one visit and MSSA from those of 268 infants (48%). Among the 32 carriers, MRSA was isolated once from 24 infants, twice from 3 infants, and three times from 5 infants.
Risk factors for MRSA carriage.
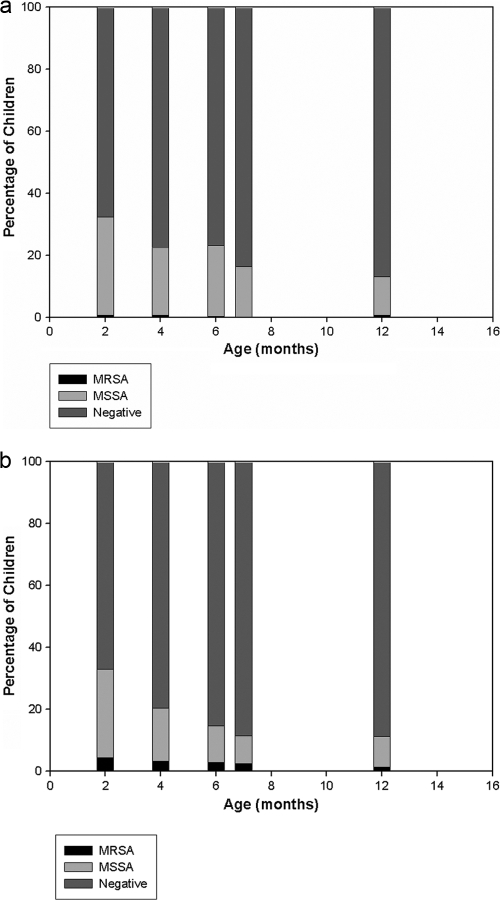
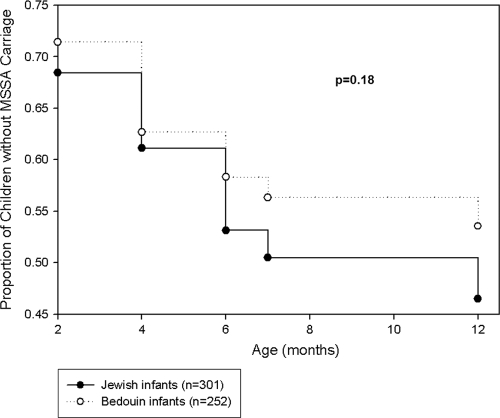
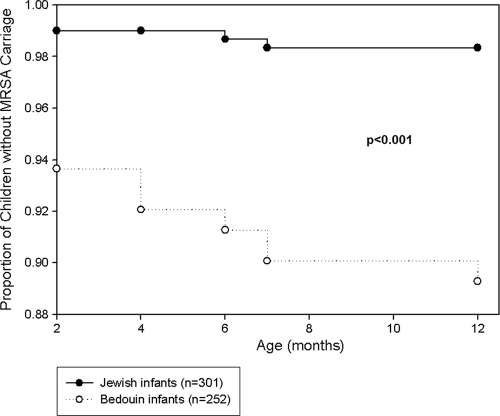
The age-specific prevalences of S. aureus carriage in Jewish and Bedouin children are presented in Fig. 1. The prevalences of MSSA carriage decreased from 167/553 (30%) at 2 months to 55/553 (10%) at 12 months and were not significantly different between Jewish and Bedouin infants. A single exception was an increase in the prevalence of MSSA carriage (65/301 to 69/301) observed for Jewish infants from the age of 4 months to the age of 6 months. However the prevalence of MRSA carriage differed significantly between the populations: rates of carriage in Bedouin infants were 11/252 (4.4%) and 6/252 (2.4%) at the ages of 2 and 12 months, respectively. In contrast, among Jewish infants, the carriage rate did not exceed 0.66%. New acquisition curves (cumulative) for MSSA and MRSA carriage according to ethnic origin are presented in Fig. 2 and 3, respectively. The risk for new acquisition of MRSA was significantly higher for Bedouin than for Jewish children (relative risk [RR], 6.62; 95% confidence interval [95% CI], 2.55 to 17.2; P < 0.001). Seven Bedouin infants carried both MRSA and MSSA at some point in time. Among the 5 Jewish infants who carried MRSA, 3 also carried MSSA.
FIG. 1.
Prevalences of S. aureus carriage among Jewish (n = 301) (a) and Bedouin (n = 252) (b) infants.
FIG. 2.
New acquisition curve (cumulative) of MSSA carriage in Jewish and Bedouin infants. The P value for ethnicity is given.
FIG. 3.
New acquisition curve (cumulative) of MRSA carriage in Jewish and Bedouin infants (RR for Bedouin infants, 6.62 [95% CI, 2.55 to 17.2]; P < 0.001).
Since only 5 Jewish children carried MRSA, further analyses were performed only with Bedouin infants in order to avoid any bias associated with ethnicity (20 Bedouin infants carried MRSA, 110 carried MSSA, and 115 carried no S. aureus). No risk factor was associated with MRSA carriage compared to MSSA carriage (the 7 infants with dual isolation of MSSA and MRSA were excluded from this analysis). The following risk factors were associated with carriage of S. aureus (MSSA and MRSA combined): previous use of antibiotics (16.8% of S. aureus carriers versus 6.1% of noncarriers; P = 0.009) and past hospitalization (13.1% of S. aureus carriers versus 3.5% of noncarriers; P = 0.007). In multivariate analysis, the only independent variable for which a significant difference was found between S. aureus carriers and noncarriers was past hospitalization (RR for S. aureus carriage, 4.2 [95% CI, 1.38 to 12.79]; P = 0.012).
Bacteriological and molecular features of MRSA strains.
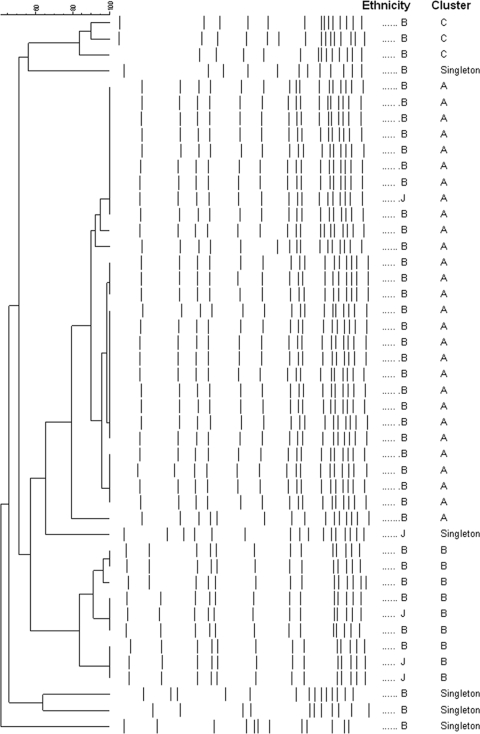
The cluster analysis of SmaI macrorestriction patterns of MRSA isolates from healthy infant carriers (n = 45) is presented in Fig. 4. Three clusters were identified. Cluster A was the most common (28/45 [62%]), followed by cluster B (9/45 [20%]), which included 3/5 Jewish children with MRSA carriage, all of whom attended the same Maternal and Child Health centers.
FIG. 4.
Cluster analysis of SmaI macrorestriction patterns of MRSA isolates from healthy infant carriers (n = 45). B, Bedouin; J, Jewish.
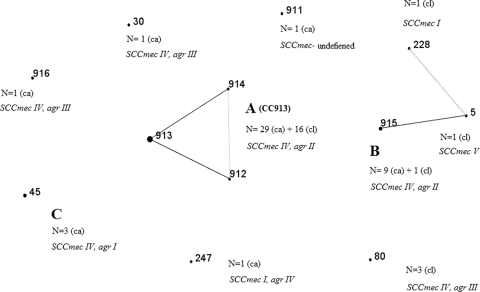
The clonal structure of MRSA isolates from healthy infant carriers is presented in Fig. 5. PFGE cluster A is a new clonal complex (CC), CC913, structured from 3 new sequence types (STs), including one from a nonrelated PFGE type (ST914). The second most common PFGE cluster, cluster B, was identified as a new ST, ST915. Two additional new STs were identified: ST911 and ST916.
FIG. 5.
Clonal structure and SCCmec and agr typing of MRSA isolates from 45 carriers (ca) and 22 clinical isolates (cl). Boldface numbers are STs. Boldface capital letters indicate the corresponding PFGE patterns (Fig. 4). Black lines indicate single-locus variations, and gray lines indicate double-locus variations.
The clinical MRSA isolates included 14 blood culture isolates and 8 wound culture isolates. One wound culture isolate was from patient 7, who also had bacteremia.
Sixteen out of the 22 clinical isolates (72%) belonged to cluster A (CC913) (PFGE data not shown). The other 6 isolates were ST915 (from cluster B) (n = 1), ST80 (n = 3), ST5 (n = 1), and ST228 (n = 1) (Fig. 5). All the main clones in the study (CC913, ST915, ST45, and ST80) carried SCCmec type IV (Fig. 5). The only clone that carried the PVL genes was ST80, and arcA was not found in any of the isolates.
Resistance to antimicrobial agents other than β-lactams was as follows: 2/22 clinical isolates were resistant to trimethoprim-sulfamethoxazole, 3/22 to gentamicin, 9/22 to clindamycin, 9/22 to erythromycin, and 3/22 to ciprofloxacin. No vancomycin-intermediate strains were found.
Clinical and epidemiological features of pediatric MRSA infections.
The clinical and demographic characteristics of pediatric MRSA infections are presented in Table 1. ST80 infections included necrotizing pneumonia, mastitis, and necrotizing soft tissue and bone infections. The last two occurred in previously healthy children with no risk factors for health care-related infections, both of them from the Bedouin township of Hura. Of note, children from this township were not part of the carriage study.
TABLE 1.
Demographic and clinical characteristics of pediatric MRSA infectionsa
| Patient no. | ST | Age | Sex | Ethnicity | Yr | Underlying condition(s) | Isolation site | Acquisition | Clinical diagnosis | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 913 | 3 mo | M | B | 2000 | Malabsorption, TPN | Blood | HA | CVC-BSI | Metastatic infection (bone) |
| 2 | 913 | 2 wk | F | B | 2001 | Prematurity | Blood | HA | CVC-BSI | None |
| 3 | 913 | 2 mo | M | B | 2004 | Short bowel, TPN | Blood | HA | CVC-BSI | None |
| 4 | 913 | 2 mo | F | B | 2005 | Infantile Crohn's disease | Blood | HA | Peripheral thrombophlebitis-related BSI | None |
| 5 | 913 | 3.5 yr | M | B | 2005 | Hyper-IgE syndrome | Blood | HA | BSI | Persistent bacteremia |
| 6 | 913 | 11 yr | M | B | 2005 | Head trauma | Blood | HA | Pneumonia | None |
| 7 | 913 | 2 yr | M | B | 2006 | Brain tumor | Blood/wound | HA | Wound-infection related BSI | None |
| 8 | 913 | 4 yr | F | B | 2006 | CIPA | Blood | HA | Osteomyelitis | Recurrence |
| 9 | 913 | 4 mo | M | B | 2006 | Congenital ichthyosis | Blood | HA | Osteomyelitis | None |
| 10 | 913 | 1 yr | M | B | 2006 | None | Wound | CA | Impetigo | None |
| 11 | 913 | 10 yr | M | B | 2006 | CIPA | Wound | HA | Stump infection | Recurrence |
| 12 | 913 | 12 yr | F | B | 2006 | CIPA | Bone | HA | Osteomyelitis | None |
| 13 | 913 | 1 yr | F | J | 2006 | Brain tumor | Wound | HA | Wound infection | None |
| 14 | 913 | 6 yr | M | B | 2006 | CIPA | Wound | HA | Stump infection | None |
| 15 | 913 | 10 yr | F | B | 2006 | None | Bone | CA | Osteomyelitis | None |
| 16 | 915 | 3 yr | M | B | 2004 | Brain tumor | Blood | HA | CVC-BSI | None |
| 17 | 80 | 2 wk | M | J | 2005 | Prematurity | Blood | HA | Pneumonia | Lung abscess |
| 18 | 80 | 3 yr | F | B | 2006 | None | Blood | CA | Osteomyelitis | Hypotension |
| 19 | 80 | 1 mo | F | B | 2006 | None | Wound | CA | Mastitis | None |
| 20 | 228 | 14 yr | M | J | 2005 | Esophageal perforation | Blood | HA | CVC-BSI | None |
| 21 | 5 | 4 yr | M | J | 2005 | Burn | Blood | HA | Burn-related BSI | None |
M, male; F, female; B, Bedouin; J, Jewish; TPN, total parenteral nutrition; CIPA, congenital insensitivity to pain with anhydrosis; HA, health care associated; CVC, central venous catheter; BSI, bloodstream infection.
In contrast, 13/15 CC913 infections occurred in children with various significant underlying diseases, and the acquisition was health care associated. Of note, 4 of these children had congenital insensitivity to pain with anhydrosis (CIPA), an inherited disorder that is relatively common among Bedouin children. These children presented with osteomyelitis or with stump infections. Only 2 cases (patients 10 and 15) were community acquired and occurred in previously healthy children. One 3.5-year-old child with hyper-IgE syndrome and chronic lung diseases had persistent CC913 bacteremia and subsequently died of Pseudomonas aeruginosa sepsis. The other MRSA infections (caused by ST5, ST228, and ST915) were hospital-acquired BSIs in children with various underlying diseases.
Relationships between isolates from this study, the 1997 outbreak, and global clones.
Five MRSA isolates from skin infections and four isolates from carriers were studied during the 1997 outbreak (4). All isolates had identical SmaI macrorestriction patterns (data not shown) and were identified as ST30. Although ST30 was also identified in one carrier in the present cohort study, the strains differed by the SCCmec subtype (IVa versus IVc) and by the presence of PVL genes in the outbreak strain but not in the carrier strain.
Although most STs in the study, including the new ST915, were related to known clonal complexes, CC913 was not (data not shown).
DISCUSSION
We found that the prevalence of MRSA nasal carriage in healthy infants in southern Israel was significantly higher in Bedouin than in Jewish infants. The prevalence of overall S. aureus carriage was comparable between the ethnic groups and decreased during the first year of life, as described by others (20, 34). The prevalence of MRSA carriage in Jewish infants in our study during the first year of life ranged from 0 to 0.66%. Previous studies reported lower prevalences of MRSA (0.15% to 0.11%) in children attending outpatient clinics in Jerusalem (39) and central Israel (36), respectively. Molecular features were studied only in the latter study (36), and only 2 of the 5 MRSA isolates (3 were isolated from adults) harbored SCCmec type IV and were identified as ST45, as was found for 3 infants in our study. On the other hand, the prevalence among Bedouin infants ranged from 4.4% to 2.4% at 2 and 12 months, respectively. This prevalence is comparable to the prevalences of 0.6% and 2.2% that have been reported from Chicago, IL (21, 42), an area with a high rate of CA-MRSA infections, and lower than the 9.2% prevalence reported from Nashville, TN (10).
Increased rates of CA-MRSA infections among semiclosed minority populations have been reported from different parts of the world, including Native Americans in the United States (18, 41) and Canada (32) and the indigenous population in Australia (47). In the Canadian (32) and Australian (47) studies, CA-MRSA isolates were polyclonal, whereas in the two studies from the United States (19, 41), the majority of isolates belonged to PFGE type USA400, suggesting dissemination from a common precursor. USA400 is also a common CA-MRSA clone in the general population in the United States. In contrast, we have found that most of the CA-MRSA isolates from Bedouin infants belonged to a single clone, designated CC913, that was not related to any known MLST clone and was isolated almost exclusively from Bedouin infants. In the Canadian study (32), it was hypothesized that the high rate of CA-MRSA infections in minority populations is related to conditions associated with poverty, such as overcrowding and high hospitalization rates. These conditions are also prevalent in the Bedouin population (data not shown). However, the overall prevalence of S. aureus carriage was not higher among Bedouin children than among Jewish children. Since Bedouin children live in crowded conditions, separately from Jewish children, it is not surprising that a single clone (CC913) was disseminated in this population and was not prominent in Jewish children.
Prior hospitalization was the only independent risk factor for overall S. aureus carriage found in our analysis. Although the association between hospitalization (18), various chronic diseases, such as diabetes, renal failure, and cirrhosis (48), and a high rate of S. aureus carriage is well known, the association with hospitalization has not been reported previously for infants without such conditions. This suggests the importance of nosocomial cross-transmission of S. aureus even in infants and young children.
Clonal complex 913 was responsible for the majority (9/14) of pediatric MRSA bloodstream infections and was the most common isolate (7/8) from wound cultures. Although our data are limited due to the relatively short duration of wound culture sampling and the lack of cultures from other sites of MRSA infection, they indicate that CC913 may now have become the main cause for pediatric MRSA infections at the Soroka University Medical Center. Surprisingly, most cases (13/15) were health care related and occurred in children with underlying diseases. In the United States and France, CA-MRSA and HA-MRSA clones were thought to be distinct from each other (46). The U.S. CA-MRSA clones differed from the HA-MRSA clones by the presence of PVL genes, SCCmec type IV, agr type III, and relative susceptibility to non-β-lactam antimicrobials. In recent years, there have been numerous reports, including reports from Israel (35), describing nosocomial outbreaks caused by strains harboring SCCmec type IV. In the United States, the USA300 pulsotype has penetrated into hospitals and is now a common cause for HA-MRSA infections in some institutions (40). In other countries (2, 8, 11, 33, 37, 46), strains harboring SCCmec type IV have been identified as major causes of HA-MRSA infections. Studying Japanese hospitals, Ma et al. (29) have demonstrated a transition in the common MRSA clones isolated, from ST30, SCCmec type IV, PVL-positive strains in 1979 to 1985 to ST5, SCCmec type II strains in the 1990s. Therefore, considering that the dominant strains in our study, CC913 and ST915 (all harboring SCCmec type IV), were isolated from healthy carriers and community-acquired infections but also from hospital-acquired infections, it is impossible to conclude whether these strains originated in or outside of the hospital environment.
The clinical spectrum of CC913 infections from our collection included mainly catheter-related bloodstream infections, osteomyelitis, and wound infections, resembling the spectrum of HA-MRSA infections (23, 30). On the other hand, the two PVL-carrying strains found in our study, ST80 and the ST30 outbreak strain (4), were characterized by severe life-threatening infections (ST80) and an outbreak of skin infections (ST30) resembling the clinical spectrum observed in CA-MRSA infections from France and the United States (13, 14, 16, 30). Whether the difference in the clinical spectrum between CC913 and ST80 infections is explained by the lack and presence of the PVL genes, respectively, goes beyond the scope of this study.
The main limitation of our study is the paucity of clinical isolates of MRSA from sources other than blood cultures, as discussed above, and the lack of clinical isolates collected in the outpatient setting. Thus, we lack data about the molecular epidemiology of the more common, less complicated community-acquired staphylococcal skin infections. Another limitation is the lack of data on exposure to possible sources of S. aureus, such as the presence of skin infections or employment of a family member in a health care facility, since this study was not initially designed to explore this question.
In conclusion, we found that a new SCCmec type IV MRSA clone, designated CC913, was the cause of most pediatric MRSA BSIs at the Soroka Hospital, as well as of the relatively high prevalence of MRSA carriage among healthy Bedouin infants. Although this clone does not seem to cause severe community-acquired infections, such as necrotizing pneumonia and soft tissue infections, its dissemination among Bedouin children may have adverse implications for therapeutic options. Further studies are required to better describe the epidemiology of community-acquired infections, to track the origin of CC913, and to describe its unique genetic characteristics.
Footnotes
Published ahead of print on 9 December 2009.
REFERENCES
- 1.Adcock, P. M., P. Pastor, F. Medley, J. E. Patterson, and T. V. Murphy. 1998. Methicillin-resistant Staphylococcus aureus in two child care centers. J. Infect. Dis. 178:577-580. [DOI] [PubMed] [Google Scholar]
- 2.Aires de Sousa, M., C. Bartzavali, I. Spiliopoulou, I. S. Sanches, M. I. Crisóstomo, and H. de Lencastre. 2003. Two international methicillin-resistant Staphylococcus aureus clones endemic in a university hospital in Patras, Greece. J. Clin. Microbiol. 41:2027-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Gurion University of the Negev. 2004. Statistical yearbook of the Negev Bedouin. Ben-Gurion University of the Negev, Beer-Sheva, Israel.
- 4.Borer, A., J. Gilad, P. Yagupski, N. Peled, N. Porat, R. Trefler, H. Shprecher-Levy, K. Riesenberg, M. Shipman, and F. Schlaeffer. 2002. Community-acquired methicillin-resistant Staphylococcus aureus in institutionalized adults with developmental disabilities. Emerg. Infect. Dis. 8:966-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 6.Central Bureau of Statistics. 1998. Statistical abstract of Israel, no. 49. Central Bureau of Statistics, Jerusalem, Israel. http://www.cbs.gov.il/archive/shnaton49/shnatone.htm.
- 7.Central Bureau of Statistics. 2005. Statistical abstract of Israel, no. 56. Central Bureau of Statistics, Jerusalem, Israel. http://www1.cbs.gov.il/reader/shnaton/shnatone_new.htm?CYear=2005&Vol=56&CSubject=26.
- 8.Chen, C. J., Y. C. Huang, C. H. Chiu, L. H. Su, and T. Y. Lin. 2005. Clinical features and genotyping analysis of community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Pediatr. Infect. Dis. J. 24:40-45. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 6th informational supplement. M100-S16; 26(3). CLSI, Wayne, PA.
- 10.Creech, C. B., II, D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M. Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617-621. [DOI] [PubMed] [Google Scholar]
- 11.Cuevas, O., E. Cercenado, E. Bouza, C. Castellares, P. Trincado, R. Cabrera, A. Vindel, and the Spanish Group for the Study of Staphylococcus. 2007. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Spain: a multicentre prevalence study (2002). Clin. Microbiol. Infect. 13:250-256. [DOI] [PubMed] [Google Scholar]
- 12.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 13.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731-739. [DOI] [PubMed] [Google Scholar]
- 14.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 15.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley for the Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 17.Goering, R. V., and M. A. Winters. 1992. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J. Clin. Microbiol. 30:577-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goslings, W. R., and K. Buchli. 1958. Nasal carrier rate of antibiotic-resistant staphylococci; influence of hospitalization on carrier rate in patients, and their household contacts. AMA Arch. Intern. Med. 102:691-715. [DOI] [PubMed] [Google Scholar]
- 19.Groom, A. V., D. H. Wolsey, T. S. Naimi, K. Smith, S. Johnson, D. Boxrud, K. A. Moore, and J. E. Cheek. 2001. Community acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201-1205. [DOI] [PubMed] [Google Scholar]
- 20.Harrison, L. M., J. A. Morris, D. R. Telford, S. M. Brown, and K. Jones. 1999. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol. Med. Microbiol. 25:19-28. [DOI] [PubMed] [Google Scholar]
- 21.Hussain, F., S. Boyle-Vavra, C. Bethel, and R. Daum. 2000. Current trends in community-acquired methicillin-resistant Staphylococcus aureus at a tertiary care pediatric facility. Pediatr. Infect. Dis. J. 19:1163-1166. [DOI] [PubMed] [Google Scholar]
- 22.Klevens, R. M., A. M. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. E. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 23.Kourbatova, E. V., J. S. Halvosa, M. D. King, S. M. Ray, N. White, and H. M. Blumberg. 2005. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 33:385-391. [DOI] [PubMed] [Google Scholar]
- 24.Larsen, A. R., S. Böcher, M. Stegger, R. Goering, L. V. Pallesen, and R. Skov. 2008. Epidemiology of European community-associated methicillin-resistant Staphylococcus aureus clonal complex 80 type IV strains isolated in Denmark from 1993 to 2004. J. Clin. Microbiol. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laupland, K. B., T. Ross, and D. B. Gregson. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J. Infect. Dis. 198:336-343. [DOI] [PubMed] [Google Scholar]
- 26.Levy, A., D. Fraser, H. Vardi, and R. Dagan. 1998. Hospitalizations for infectious diseases in Jewish and Bedouin children in southern Israel. Eur. J. Epidemiol. 14:179-186. [DOI] [PubMed] [Google Scholar]
- 27.Lina, G., Y. Piémont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 28.Lina, G., F. Boutite, A. Tristan, M. Bes, J. Etienne, and F. Vandenesch. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environ. Microbiol. 69:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, X. X., T. Ito, P. Chongtrakool, and K. Hiramatsu. 2006. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 44:4515-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien, F. G., J. W. Pearman, M. Gracey, T. V. Riley, and W. B. Grubb. 1999. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J. Clin. Microbiol. 37:2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofner-Agostini, M., A. E. Simor, M. Mulvey, E. Bryce, M. Loeb, A. McGeer, A. Kiss, and S. Paton; Canadian Nosocomial Infection Surveillance Program, Health Canada. 2006. Methicillin-resistant Staphylococcus aureus in Canadian aboriginal people. Infect. Control Hosp. Epidemiol. 27:204-207. [DOI] [PubMed] [Google Scholar]
- 33.Ramdani-Bouguessa, N., M. Bes, H. Meugnier, F. Forey, M. E. Reverdy, G. Lina, F. Vandenesch, M. Tazir, and J. Etienne. 2006. Detection of methicillin-resistant Staphylococcus aureus strains resistant to multiple antibiotics and carrying the Panton-Valentine leukocidin genes in an Algiers hospital. Antimicrob. Agents Chemother. 50:1083-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regev-Yochay, G., R. Dagan, M. Raz, Y. Carmeli, B. Shainberg, E. Derazne, G. Rahav, and E. Rubinstein. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292:716-720. [DOI] [PubMed] [Google Scholar]
- 35.Regev-Yochay, G., E. Rubinstein, A. Barzilai, Y. Carmeli, J. Kuint, J. Etienne, M. Blech, G. Smollen, A. Maayan-Metzger, A. Leavitt, G. Rahav, and N. Keller. 2005. Methicillin-resistant Staphylococcus aureus in neonatal intensive care unit. Emerg. Infect. Dis. 11:453-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regev-Yochay, G., Y. Carmeli, M. Raz, E. Pinco, J. Etienne, A. Leavitt, E. Rubinstein, and S. Navon-Venezia. 2006. Prevalence and genetic relatedness of community-acquired methicillin-resistant Staphylococcus aureus in Israel. Eur. J. Clin. Microbiol. Infect. Dis. 25:719-722. [DOI] [PubMed] [Google Scholar]
- 37.Rossney, A. S., M. J. Lawrence, P. M. Morgan, M. M. Fitzgibbon, A. Shore, D. C. Coleman, C. T. Keane, and B. O'Connell. 2006. Epidemiological typing of MRSA isolates from blood cultures taken in Irish hospitals participating in the European Antimicrobial Resistance Surveillance System (1999-2003). Eur. J. Clin. Microbiol. Infect. Dis. 25:79-89. [DOI] [PubMed] [Google Scholar]
- 38.Saiman, L., M. O'Keefe, P. L. Graham III, F. Wu, B. Saïd-Salim, B. Kreiswirth, A. LaSala, P. M. Schlievert, and P. Della-Latta. 2003. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin. Infect. Dis. 37:1313-1319. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger, Y., S. Yahalom, D. Raveh, A. M. Yinnon, R. Segel, M. Erlichman, D. Attias, and B. Rudensky. 2003. Methicillin-resistant Staphylococcus aureus nasal colonization in children in Jerusalem: community vs. chronic care institutions. Isr. Med. Assoc. J. 5:847-851. [PubMed] [Google Scholar]
- 40.Seybold, U., E. V. Kourbatova, J. G. Johnson, S. J. Halvosa, Y. F. Wang, M. D. King, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647-656. [DOI] [PubMed] [Google Scholar]
- 41.Stemper, M. E., S. K. Shukla, and K. D. Reed. 2004. Emergence and spread of community-associated methicillin-resistant Staphylococcus aureus in rural Wisconsin, 1989 to 1999. J. Clin. Microbiol. 42:5673-5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suggs, A., M. Maranan, S. Boyle-Vavra, and R. Daum. 1999. Methicillin-resistant and borderline methicillin-resistant asymptomatic Staphylococcus aureus colonization in children without identifiable risk factors. Pediatr. Infect. Dis. J. 18:410-414. [DOI] [PubMed] [Google Scholar]
- 43.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiemersma, E. W., S. L. Bronzwaer, O. Lyytikäinen, J. E. Degener, P. Schrijnemakers, N. Bruinsma, J. Monen, W. Witte, H. Grundman, and European Antimicrobial Resistance Surveillance System participants. 2004. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg. Infect. Dis. 10:1627-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnidge, J. D., and J. M. Bell. 2000. Methicillin-resistant Staphylococcal aureus evolution in Australia over 35 years. Microb. Drug Resist. 6:223-229. [DOI] [PubMed] [Google Scholar]
- 46.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlack, S., L. Cox, A. Y. Peleg, C. Canuto, C. Stewart, A. Conlon, A. Stephens, P. Giffard, F. Huygens, A. Mollinger, R. Vohra, and J. S. McCarthy. 2006. Carriage of methicillin-resistant Staphylococcus aureus in a Queensland indigenous community. Med. J. Aust. 184:556-559. [DOI] [PubMed] [Google Scholar]
- 48.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, K., J. A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinderman, C. E., B. Conner, M. A. Malakooti, J. E. LaMar, A. Armstrong, and B. K. Bohnker. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]