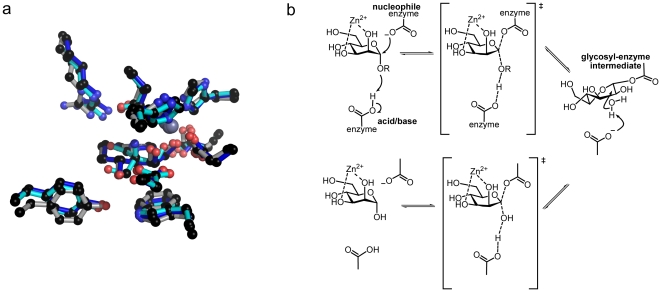
Figure 5. Conservation of GH38 reaction mechanism.
(A) Conserved active-centre constellation (here -1 subsite only) between the SpGH38 (grey), bovine bLAM (cyan) and the Drosophila GH38 α−mannosidase (blue). (B). GH38 α−mannosidases are known to act with net retention of anomeric configuration; a mechanism in which a glycosyl-enzyme intermediate is flanked by oxocarbenium-ion like transition-states. The intermediate has been trapped by the Withers and Rose groups [17] and shown to bind in a 1S5 skew-boat conformation which in the absence of evidence to the contrary might imply a transition-state close to a B2,5. Pseudo-Michaelis complexes published on the Drosophila α−mannosidase II show the −1 sugar in a 4C1 chair conformation but these have been obtained on a nucleophile-alanine variant so their conformational relevance to catalysis is unclear [15].