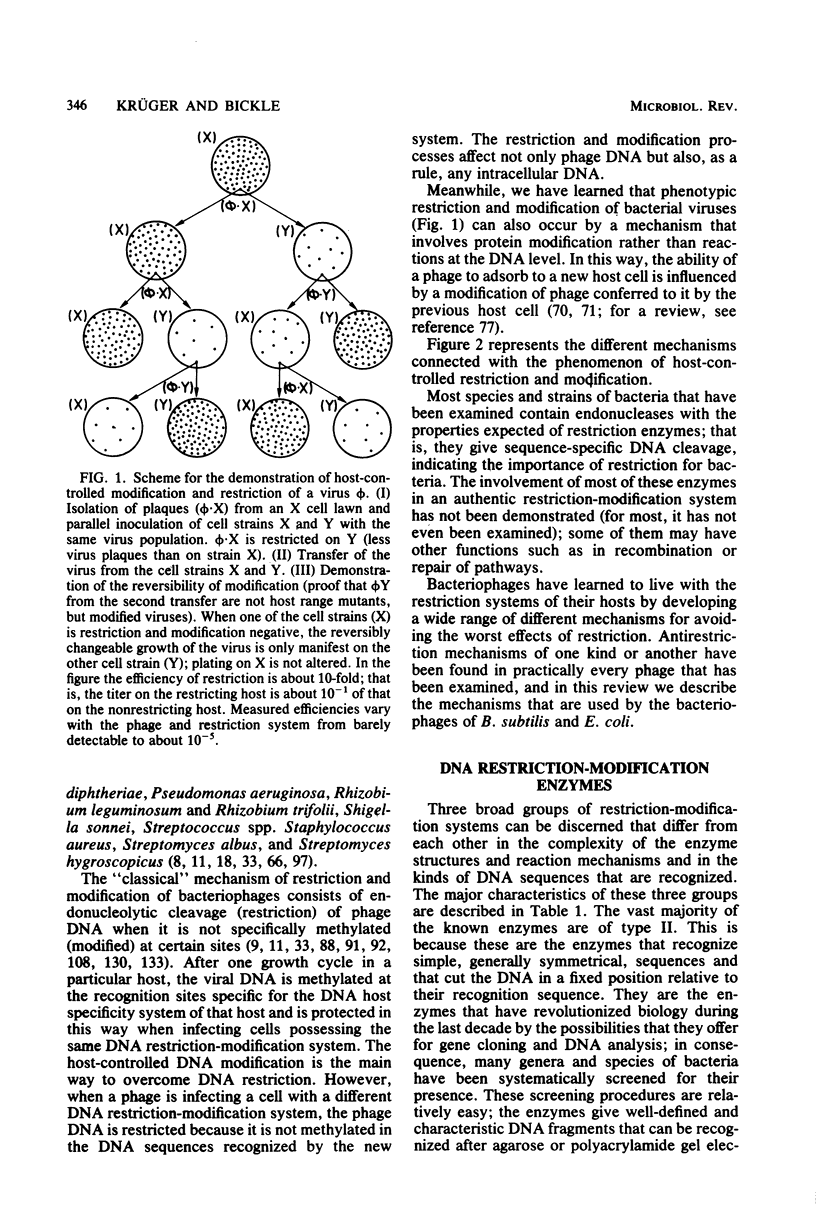
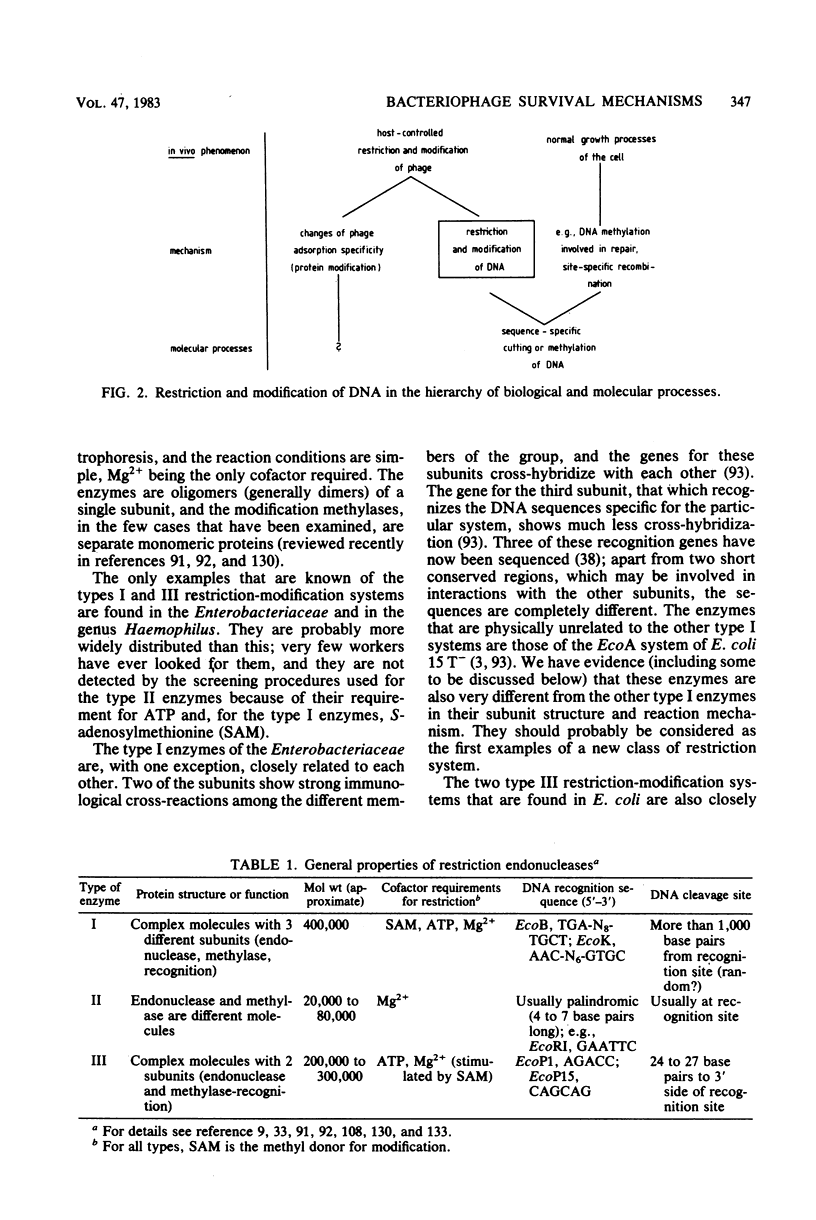
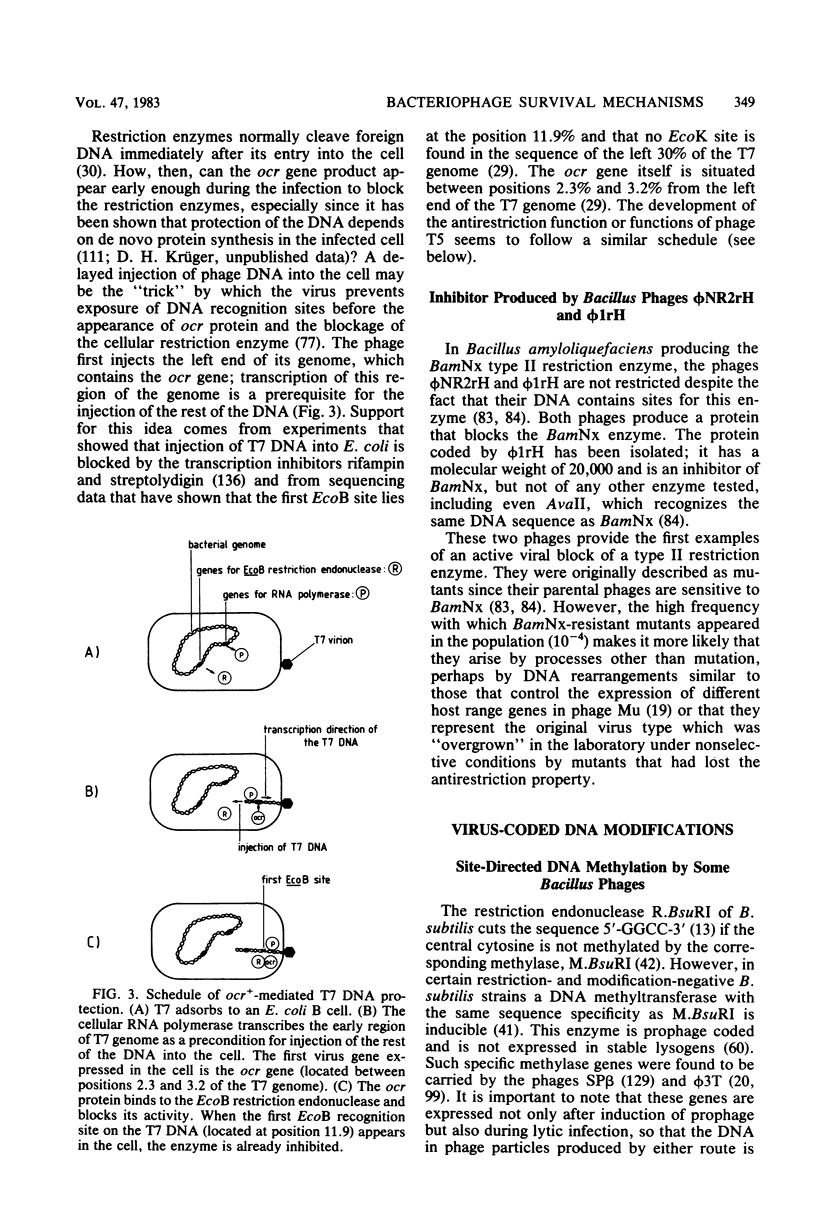
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Kühnlein U. Mutationeller Verlust B-spezifischer Restriktion des Bakteriophagen fd. Pathol Microbiol (Basel) 1967;30(6):946–952. [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. The effects of substituted pyrimidines in DNAs on cleavage by sequence-specific endonucleases. J Biol Chem. 1979 Apr 10;254(7):2551–2560. [PubMed] [Google Scholar]
- Bernheimer H. P. Lysogenic pneumococci and their bacteriophages. J Bacteriol. 1979 May;138(2):618–624. doi: 10.1128/jb.138.2.618-624.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T., Arber W. Host-controlled restriction and modification of filamentous 1- and F-specific bacteriophages. Virology. 1969 Nov;39(3):605–607. doi: 10.1016/0042-6822(69)90112-3. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Bron S., Luxen E., Trautner T. A. Restriction and modification in B. subtilis: the role of homology between donor and recipient DNA in transformation and transfection. Mol Gen Genet. 1980;179(1):111–117. doi: 10.1007/BF00268452. [DOI] [PubMed] [Google Scholar]
- Bron S., Murray K. Restriction and modification in B. subtilis. Nucleotide sequence recognised by restriction endonuclease R. Bsu R from strain R. Mol Gen Genet. 1975 Dec 30;143(1):25–33. doi: 10.1007/BF00269417. [DOI] [PubMed] [Google Scholar]
- Brooks J. E., Hattman S. In vitro methylation of bacteriophage lambda DNA by wild type (dam+) and mutant (damh) forms of the phage T2 DNA adenine methylase. J Mol Biol. 1978 Dec 15;126(3):381–394. doi: 10.1016/0022-2836(78)90047-5. [DOI] [PubMed] [Google Scholar]
- Brunel F., Davison J. Restriction insensitivity in bacteriophage T5. III. Characterization of EcoRI-sensitive mutants by restriction analysis. J Mol Biol. 1979 Mar 15;128(4):527–543. doi: 10.1016/0022-2836(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Burckhardt J., Weisemann J., Yuan R. Characterization of the DNA methylase activity of the restriction enzyme from Escherichia coli K. J Biol Chem. 1981 Apr 25;256(8):4024–4032. [PubMed] [Google Scholar]
- Bächi B., Reiser J., Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J Mol Biol. 1979 Feb 25;128(2):143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg J. M., Nguyen A. H., Ito J. DNA modification induced during infection of Bacillus subtilis by phage phi 3T. Gene. 1980 Dec;12(1-2):17–24. doi: 10.1016/0378-1119(80)90011-6. [DOI] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- Davison J., Brunel F. Restriction insensitivity in bacteriophage T5 I. Genetic characterization of mutants sensitive to EcoRI restriction. J Virol. 1979 Jan;29(1):11–16. doi: 10.1128/jvi.29.1.11-16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J., Brunel F. Restriction insensitivity in bacteriophage T5. II. Lack of EcoRI modification in T5+ and T5ris mutants. J Virol. 1979 Jan;29(1):17–20. doi: 10.1128/jvi.29.1.17-20.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrouwere L., Van Montagu M., Schell J. The ral gene of phage lambda. III. Interference with E. coli ATP dependent functions. Mol Gen Genet. 1980;179(1):81–88. doi: 10.1007/BF00268449. [DOI] [PubMed] [Google Scholar]
- Debrouwere L., Zabeau M., Van Montagu M., Schell J. The ral gene of phage lambda. II. Isolation and characterization of ral deficient mutants. Mol Gen Genet. 1980;179(1):75–80. doi: 10.1007/BF00268448. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Mechanism localisation and control of restriction cleavage of phage T4 and lambda chromosomes in vivo. Nature. 1976 Apr 1;260(5550):406–410. doi: 10.1038/260406a0. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Phage-coded protein prevents restriction of unmodified progeny T4 DNA. Nature. 1976 Apr 1;260(5550):454–456. doi: 10.1038/260454a0. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Revel H. R., Goldberg E. B. Physical mapping and cloning of bacteriophage T4 anti-restriction endonuclease gene. J Bacteriol. 1982 Feb;149(2):694–699. doi: 10.1128/jb.149.2.694-699.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981 Nov;57(Pt 1):1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Elliott J., Arber W. E. coli K-12 pel mutants, which block phage lambda DNA injection, coincide with ptsM, which determines a component of a sugar transport system. Mol Gen Genet. 1978 Apr 25;161(1):1–8. doi: 10.1007/BF00266608. [DOI] [PubMed] [Google Scholar]
- Eskridge R. W., Weinfeld H., Paigen K. Susceptibility of different coliphage genomes to host-controlled variation. J Bacteriol. 1967 Mar;93(3):835–844. doi: 10.1128/jb.93.3.835-844.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Ganesan A. K., Minton K. N-Glycosidase activity in extracts of Bacillus subtilis and its inhibition after infection with bacteriophage PBS2. J Virol. 1975 Aug;16(2):315–321. doi: 10.1128/jvi.16.2.315-321.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M., Gefter M., Hausmann R., Hurwitz J. Methylation of DNA. J Gen Physiol. 1966 Jul;49(6):5–28. doi: 10.1085/jgp.49.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Günthert U., Freund M., Trautner T. A. Restriction and modification in Bacillus subtilis: two DNA methyltransferases with BsuRI specificity. I. Purification and physical properties. J Biol Chem. 1981 Sep 10;256(17):9340–9345. [PubMed] [Google Scholar]
- Günthert U., Jentsch S., Freund M. Restriction and modification in Bacillus subtilis: two DNA methyltransferases with BsuRI specificity. II. Catalytic properties, substrate specificity, and mode of action. J Biol Chem. 1981 Sep 10;256(17):9346–9351. [PubMed] [Google Scholar]
- Günthert U., Pawlek B., Stutz J., Trautner T. A. Restriction and modification in Bacillus subtilis: inducibility of a DNA methylating activity in nonmodifying cells. J Virol. 1976 Oct;20(1):188–195. doi: 10.1128/jvi.20.1.188-195.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günthert U., Storm K., Bald R. Restriction and modification in Bacillus subtilis. Localization of the methylated nucleotide in the BsuRI recognition sequence. Eur J Biochem. 1978 Oct 16;90(3):581–583. doi: 10.1111/j.1432-1033.1978.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Bächi B., Iida S., Bickle T. A. DNA restriction--modification enzymes of phage P1 and plasmid p15B. Subunit functions and structural homologies. J Mol Biol. 1983 Mar 25;165(1):19–34. doi: 10.1016/s0022-2836(83)80240-x. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Bächi B., Shepherd J. C., Yuan R., Ineichen K., Bickle T. A. DNA recognition and cleavage by the EcoP15 restriction endonuclease. J Mol Biol. 1979 Nov 5;134(3):655–666. doi: 10.1016/0022-2836(79)90372-3. [DOI] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Hattman S. DNA methylation of T-even bacteriophages and of their nonglucosylated mutants: its role in P1-directed restriction. Virology. 1970 Oct;42(2):359–367. doi: 10.1016/0042-6822(70)90279-5. [DOI] [PubMed] [Google Scholar]
- Hattman S. DNA methyltransferase-dependent transcription of the phage Mu mom gene. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5518–5521. doi: 10.1073/pnas.79.18.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S. Specificity of the bacteriophage Mu mom+ -controlled DNA modification. J Virol. 1980 Apr;34(1):277–279. doi: 10.1128/jvi.34.1.277-279.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S. Unusual modification of bacteriophage Mu DNA. J Virol. 1979 Nov;32(2):468–475. doi: 10.1128/jvi.32.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., van Ormondt H., de Waard A. Sequence specificity of the wild-type dam+) and mutant (damh) forms of bacteriophage T2 DNA adenine methylase. J Mol Biol. 1978 Mar 5;119(3):361–376. doi: 10.1016/0022-2836(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Farnet C. M., Ehrlich K. C., Ehrlich M. Digestion of highly modified bacteriophage DNA by restriction endonucleases. Nucleic Acids Res. 1982 Mar 11;10(5):1579–1591. doi: 10.1093/nar/10.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Meyer J., Bächi B., Stålhammar-Carlemalm M., Schrickel S., Bickle T. A., Arber W. DNA restriction--modification genes of phage P1 and plasmid p15B. Structure and in vitro transcription. J Mol Biol. 1983 Mar 25;165(1):1–18. doi: 10.1016/s0022-2836(83)80239-3. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Matsumoto K., Iijima T., Saito H., Ando T. Chromosomal loci of genes controlling site-specific restriction endonucleases of Bacillus subtilis. Mol Gen Genet. 1981;183(1):1–6. doi: 10.1007/BF00270129. [DOI] [PubMed] [Google Scholar]
- Ineichen K., Shepherd J. C., Bickle T. A. The DNA sequence of the phage lambda genome between PL and the gene bet. Nucleic Acids Res. 1981 Sep 25;9(18):4639–4653. doi: 10.1093/nar/9.18.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Roberts R. J. Unusual base sequence arrangement in phage phi 29 DNA. Gene. 1979 Jan;5(1):1–7. doi: 10.1016/0378-1119(79)90088-x. [DOI] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Karran P., Cone R., Friedberg E. C. Specificity of the bacteriophage PBS2 induced inhibitor of uracil-DNA glycosylase. Biochemistry. 1981 Oct 13;20(21):6092–6096. doi: 10.1021/bi00524a027. [DOI] [PubMed] [Google Scholar]
- Katz G. E., Price A. R., Pomerantz M. J. Bacteriophage PBS2-induced inhibition of uracil-containing DNA degradation. J Virol. 1976 Nov;20(2):535–538. doi: 10.1128/jvi.20.2.535-538.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Mizukami T., Shimotsu H., Anzai H., Takahashi H., Saito H. Unusually infrequent cleavage with several endonucleases and physical map construction of Bacillus subtilis bacteriophage phi 1 DNA. J Virol. 1981 Mar;37(3):1099–1102. doi: 10.1128/jvi.37.3.1099-1102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon H., Bukhari A. I. Bacteriophage Mu-induced modification of DNA is dependent upon a host function. J Bacteriol. 1978 Oct;136(1):423–428. doi: 10.1128/jb.136.1.423-428.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S., Hartmann M., Krügel H., Roth M., Walter F., Rautenstein Y. I., Solovyeva N. Y. Restriction of streptomyces phage SH5 by endonuclease ShyI from Streptomyces hygroscopicus 0477. Mol Gen Genet. 1981;184(2):286–288. doi: 10.1007/BF00272919. [DOI] [PubMed] [Google Scholar]
- Krueger D. H., Presber W., Hansen S., Rosenthal H. A. Biological functions of the bacteriophage T3 SAMase gene. J Virol. 1975 Aug;16(2):453–455. doi: 10.1128/jvi.16.2.453-455.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Chernin L. S., Hansen S., Rosenthal H. A., Goldfarb D. M. Protection of foreign DNA against host-controlled restriction in bacterial cells. I. protection of F' plasmid DNA by preinfection with bacteriophages T3 or T7. Mol Gen Genet. 1978 Feb 7;159(1):107–110. doi: 10.1007/BF00401754. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Gola G., Weisshuhn I., Hansen S. The ocr gene function of bacterial viruses T3 and T7 prevents host-controlled modification. J Gen Virol. 1978 Oct;41(1):189–192. doi: 10.1099/0022-1317-41-1-189. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Reuter M. The ocr+ gene function of bacteriophages T3 and T7 counteracts the Salmonella typhimurium DNA restriction systems SA and SB. J Virol. 1983 Mar;45(3):1147–1149. doi: 10.1128/jvi.45.3.1147-1149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C. Host-dependent modification of bacterial virus T3 affecting its adsorption ability. Virology. 1980 Apr 30;102(2):444–446. doi: 10.1016/0042-6822(80)90111-7. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C. Virus adaptation to host cells: the non-classical modification of phage T3. Z Allg Mikrobiol. 1980;20(8):495–502. [PubMed] [Google Scholar]
- Krüger D. H., Presber W., Hansen S., Rosenthal H. A. Different restriction of bacteriophages T3 and T7 by P1-lysogenic cells and the role of the T3-coded SAMase. Z Allg Mikrobiol. 1977;17(8):581–591. doi: 10.1002/jobm.3630170802. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Reuter M., Hansen S., Schroeder C. Influence of phage T3 and T7 gene functions on a type III(EcoP1) DNA restriction-modification system in vivo. Mol Gen Genet. 1982;185(3):457–461. doi: 10.1007/BF00334140. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Reuter M., Schroeder C., Glatman L. I., Chernin L. S. Restriction of bacteriophage T3 and T7 ocr+ strains by the type II restriction endonuclease EcoRV. Mol Gen Genet. 1983;190(2):349–351. doi: 10.1007/BF00330663. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C., Hansen S., Rosenthal H. A. Active protection by bacteriophages T3 and T7 against E. coli B- and K-specific restriction of their DNA. Mol Gen Genet. 1977 May 20;153(1):99–106. doi: 10.1007/BF01036001. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Lark C., Arber W. Host specificity of DNA produced by Escherichia coli. 13. Breakdown of cellular DNA upon growth in ethionine of strains with r plus-15, r plus-P1 or r plus-N3 restriction phenotypes. J Mol Biol. 1970 Sep 14;52(2):337–348. doi: 10.1016/0022-2836(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Makino O., Kawamura J., Saito H., Ikeda Y. Inactivation of restriction endonuclease bamNx after infection with phage phi NR2. Nature. 1979 Jan 4;277(5691):64–66. doi: 10.1038/277064a0. [DOI] [PubMed] [Google Scholar]
- Makino O., Saito H., Ando T. Bacillus subtilis-phage phi 1 overcomes host-controlled restriction by producing BamNx inhibitor protein. Mol Gen Genet. 1980;179(3):463–468. doi: 10.1007/BF00271735. [DOI] [PubMed] [Google Scholar]
- Mark K. K., Studier F. W. Purification of the gene 0.3 protein of bacteriophage T7, an inhibitor of the DNA restriction system of Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2573–2578. [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. The effect of sequence specific DNA methylation on restriction endonuclease cleavage. Nucleic Acids Res. 1981 Nov 25;9(22):5859–5866. doi: 10.1093/nar/9.22.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Fujisawa H., Ryo Y., Minagawa T. Isolation and characterization of bacteriophage T3 mutants sensitive to restriction by EcoRI. Virology. 1982 Oct 15;122(1):1–7. doi: 10.1016/0042-6822(82)90371-3. [DOI] [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Gough J. A., Suri B., Bickle T. A. Structural homologies among type I restriction-modification systems. EMBO J. 1982;1(5):535–539. doi: 10.1002/j.1460-2075.1982.tb01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol'skaia I. I., Tediashvili M. G., Vasil'eva M. B., Chanishvili T. G., Debov S. S. Izuchenie sistem khoziaiskoi spetsifichnosti i metilaz DNK u shigell i ikh fagov. Vopr Virusol. 1978 Nov-Dec;(6):724–731. [PubMed] [Google Scholar]
- Nikolskaya I. I., Lopatina N. G., Chaplygina N. M., Debov S. S. The host specificity system in Escherichia coli SK. Mol Cell Biochem. 1976 Nov 30;13(2):79–87. doi: 10.1007/BF01837057. [DOI] [PubMed] [Google Scholar]
- Nikolskaya I. I., Tediashvili M. I., Lopatina N. G., Chanishvili T. G., Debov S. S. Specificity and functions of guanine methylase of Shigella sonnei DDVI phage. Biochim Biophys Acta. 1979 Jan 26;561(1):232–239. doi: 10.1016/0005-2787(79)90506-9. [DOI] [PubMed] [Google Scholar]
- Noyer-Weidner M., Jentsch S., Pawlek B., Günthert U., Trautner T. A. Restriction and modification in Bacillus subtilis: DNA methylation potential of the related bacteriophages Z, SPR, SP beta, phi 3T, and rho 11. J Virol. 1983 May;46(2):446–453. doi: 10.1128/jvi.46.2.446-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer-Weidner M., Pawlek B., Jentsch S., Günthert U., Trautner T. A. Restriction and modification in Bacillus subtilis: gene coding for a BsuR-specific modification methyltransferase in the temperate bacteriophage phi 3T. J Virol. 1981 Jun;38(3):1077–1080. doi: 10.1128/jvi.38.3.1077-1080.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarowicz A., Bickle T. A., Shepherd J. C., Ineichen K. The DNA sequence recognised by the HinfIII restriction endonuclease. J Mol Biol. 1981 Feb 15;146(1):167–172. doi: 10.1016/0022-2836(81)90372-7. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Vrieling H., Van de Putte P. Transcription initiation of Mu mom depends on methylation of the promoter region and a phage-coded transactivator. Nature. 1983 Jan 27;301(5898):344–347. doi: 10.1038/301344a0. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Amann E., Tailor R., Günthert U., Scholz K., Trautner T. A. Unusual behaviour of SPO1 DNA with respect to restriction and modification enzymes recognizing the sequence 5'-G-G-C-C. Mol Gen Genet. 1980 Apr;178(1):229–231. doi: 10.1007/BF00267234. [DOI] [PubMed] [Google Scholar]
- Reuter M., Krüger D. H., Scholz D., Rosenthal H. A. Schutz zellfremder DNA vor wirtskontrollierter Restriktion in Bakterienzellen. II. Schutz des Plasmids pSF2124 durch die ocr+ Genfunktion der Bakteriophagen T3 und T7. Z Allg Mikrobiol. 1980;20(5):345–354. doi: 10.1002/jobm.3630200506. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Georgopoulos C. P. Restriction of nonglucosylated T-even bacteriophages by prophage P1. Virology. 1969 Sep;39(1):1–17. doi: 10.1016/0042-6822(69)90343-2. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Rhoades M. Cleavage of T5 DNA by the Escherichia coli R-I restriction endonuclease. Virology. 1975 Mar;64(1):170–179. doi: 10.1016/0042-6822(75)90089-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Simon M. N., Studier F. W., Roberts R. J. Survey and mapping of restriction endonuclease cleavage sites in bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):907–915. doi: 10.1016/0022-2836(79)90519-9. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Satta G., Debbia E., Pruzzo C., Calegari L. The peculiar behaviour of coliphage P1vir mutants on restricting hosts. Microbios. 1978;22(88):93–102. [PubMed] [Google Scholar]
- Scandella D., Arber W. An Escherichia coli mutant which inhibits the injection of phage lambda DNA. Virology. 1974 Apr;58(2):504–513. doi: 10.1016/0042-6822(74)90084-1. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. Phage lambda DNA injection into Escherichia coli pel- mutants is restored by mutations in phage genes V or H. Virology. 1976 Jan;69(1):206–215. doi: 10.1016/0042-6822(76)90207-5. [DOI] [PubMed] [Google Scholar]
- Shepherd J. C. Periodic correlations in DNA sequences and evidence suggesting their evolutionary origin in a comma-less genetic code. J Mol Evol. 1981;17(2):94–102. doi: 10.1007/BF01732679. [DOI] [PubMed] [Google Scholar]
- Sommer R., Schaller H. Nucleotide sequence of the recognition site of the B-specific restriction modification system in E. coli. Mol Gen Genet. 1979 Jan 11;168(3):331–335. doi: 10.1007/BF00271504. [DOI] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P., Bickle T. A. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature. 1979 Mar 1;278(5699):30–34. doi: 10.1038/278030a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Jan;78(1):205–209. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita F. Changes in DNase activities in Bacillus subtilis infected with bacteriophage PBS 1. J Virol. 1975 May;15(5):1073–1080. doi: 10.1128/jvi.15.5.1073-1080.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A. DNA modification of bacteriophage Mu-1 requires both host and bacteriophage functions. J Virol. 1977 Sep;23(3):825–826. doi: 10.1128/jvi.23.3.825-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A., Desmet L., Faelen M. Mapping of the modification function of temperate phage Mu-1. Mol Gen Genet. 1980 Jan;177(2):351–353. doi: 10.1007/BF00267450. [DOI] [PubMed] [Google Scholar]
- Toussaint A. The DNA modification function of temperate phage Mu-1. Virology. 1976 Mar;70(1):17–27. doi: 10.1016/0042-6822(76)90232-4. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Pawlek B., Günthert U., Canosi U., Jentsch S., Freund M. Restriction and modification in Bacillus subtilis: identification of a gene in the temperate phage SP beta coding for a BsuR specific modification methyltransferase. Mol Gen Genet. 1980;180(2):361–367. doi: 10.1007/BF00425849. [DOI] [PubMed] [Google Scholar]
- Vovis G. F., Horiuchi K., Zinder N. D. Kinetics of methylation of DNA by a restriction endonuclease from Escherichia coli B. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3810–3813. doi: 10.1073/pnas.71.10.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. A new pyrimidine base from bacteriophage nucleic acids. Nature. 1952 Dec 20;170(4338):1072–1073. doi: 10.1038/1701072a0. [DOI] [PubMed] [Google Scholar]
- Warren R. A. Modified bases in bacteriophage DNAs. Annu Rev Microbiol. 1980;34:137–158. doi: 10.1146/annurev.mi.34.100180.001033. [DOI] [PubMed] [Google Scholar]
- Witmer H., Franks M. Restriction and modification of bacteriophage SP10 DNA by Bacillus subtilis Marburg 168: stabilization of SP10 DNA in restricting hosts preinfected with a heterologous phage, SP18. J Virol. 1981 Jan;37(1):148–155. doi: 10.1128/jvi.37.1.148-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Bickle T. A., Ebbers W., Brack C. Multiple steps in DNA recognition by restriction endonuclease from E. coli K. Nature. 1975 Aug 14;256(5518):556–560. doi: 10.1038/256556a0. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Shemyakin M. F. RNA polymerase-dependent mechanism for the stepwise T7 phage DNA transport from the virion into E. coli. Nucleic Acids Res. 1982 Mar 11;10(5):1635–1652. doi: 10.1093/nar/10.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Hayward G. S., Bujard H. Physical mapping of the HindIII, EcoRI, Sal and Sma restriction endonuclease cleavage fragments from bacteriophage T5 DNA. Mol Gen Genet. 1976 Feb 2;143(3):279–290. doi: 10.1007/BF00269404. [DOI] [PubMed] [Google Scholar]


