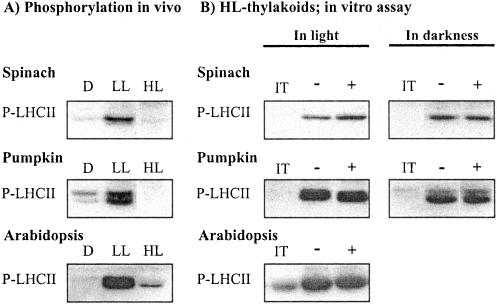
Figure 1.
A, Irradiance-dependent phosphorylation of LHCII proteins in leaves. Plant leaves were incubated in darkness (D) or illuminated at a low (LL) and high (HL) light for2has described in “Materials and Methods.” The leaf samples were frozen in liquid nitrogen, and the phosphorylation level of LHCII proteins in isolated thylakoids was analyzed by immunoblotting. B, Restoration of LHCII protein phosphorylation capacity in vitro in thylakoid membranes isolated from high-light-illuminated leaves. Isolated thylakoid membranes were incubated in darkness for 10 min in the absence (-) or the presence of 2 mm DTTox (+), and subsequently LHCII protein phosphorylation was initiated by the addition of ATP and switching the light on. Phosphorylation of LHCII proteins was also carried out in darkness by activating the kinase with NADPH and ferredoxin. Phosphoproteins were detected by immunoblotting with phospho-Thr antibody. IT indicates phosphorylation level of LHCII proteins in isolated thylakoids before the phosphorylation assay. For quantification of the data, see Table I.