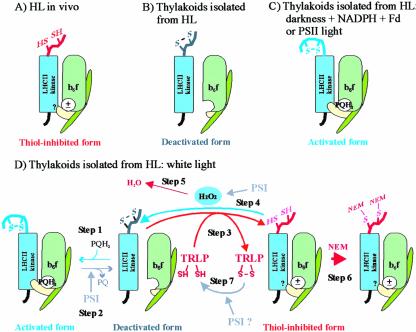
Figure 7.
Schematic presentation of the activation states of the LHCII kinase in thylakoid membranes of high-light-illuminated leaves (A) and in thylakoids isolated from such leaves and treated experimentally as presented in this paper (B-D). A, A thiol-inhibited form of the LHCII kinase predominates in leaves under high redox potential in chloroplasts in vivo. B, The thiol-inhibited LHCII kinase is oxidized during isolation of thylakoids from high-light-illuminated leaves (HL). In these membranes, the plastoquinone pool is oxidized, and thus the LHCII kinase is in deactivated form. C, Reduction of the plastoquinone pool and subsequent binding of plastoquinol (PQH2) to the Qo site of Cyt b6f complex induces activation of the LHCII kinase. The activated form of the LHCII kinase predominates in thylakoid membranes illuminated in PSII light or incubated in darkness in the presence of NADPH and ferredoxin (see “Discussion”). D, Dynamic activation/deactivation and thiol-induced inhibition of the LHCII kinase in thylakoids illuminated in white light. The steps of the model, marked 1 to 7, are described in the “Discussion.” The movement of Rieske protein (yellow subunit of Cyt b6f complex) between proximal and distal positions with respect to the membrane, induced by binding/release of plastoquinone to/from the Qo site, respectively (Darrouzet et al., 2000; Wollman, 2001), is shown. ±, The dithiol form of the LHCII kinase is inhibited despite the presence and absence of PQH2 at the Qo site of Cyt b6f complex.? in the LHCII kinase, Interaction of the thiol-inhibited LHCII kinase with Cyt b6f complex is unknown. TRLP, Thioredoxin-like protein. For simplicity, only the monomeric form of the Cyt b6f complex is presented, and the membrane bilayer structure is not shown.