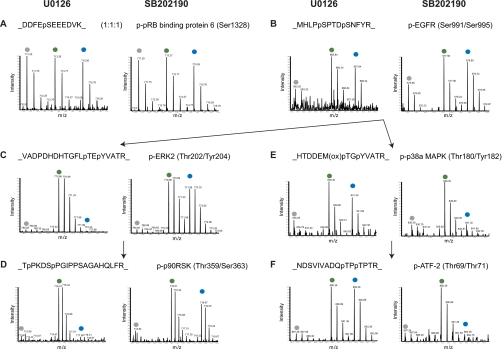
Fig. 2.
Effects of the inhibitors U0126 and SB202190 on known substrates of signaling branches of ERK1/2 and p38α/β. MS survey scans present phosphopeptide intensities in each of the SILAC triplets. Peaks marked on top by gray, green, and blue dots represent phosphopeptides from light, medium, and heavy labeled cells, respectively. For labeling details see “Experimental Procedures.” In each panel, the figure on the left is from the “U0126 experiment,” whereas the figure on the right is from the “SB202190 experiment.” A, a phosphopeptide was affected by neither the EGF stimulus nor the two inhibitors, demonstrating a 1:1:1 ratio. B, a phosphoserine peptide of EGF receptor (EGFR) was highly activated by EGF but not responsive to the two inhibitors. C, the autophosphorylation sites of ERK2 were highly activated by EGF and suppressed by U0126 but not responsive to SB202190. D, a known substrate of ERK1/2 demonstrated the same response pattern as the ERK1/2 autophosphorylation. E, the autophosphorylation sites of p38α were highly activated by EGF and suppressed by SB202190 but not responsive to U0126. F, a known substrate of p38α demonstrated the same response pattern as the p38α autophosphorylation. ox, oxidation; p-, phospho-; pRB, phospho-retinoblastoma; p90RSK, 90 kDa ribosomal protein S6 kinase.