Fig. 5.
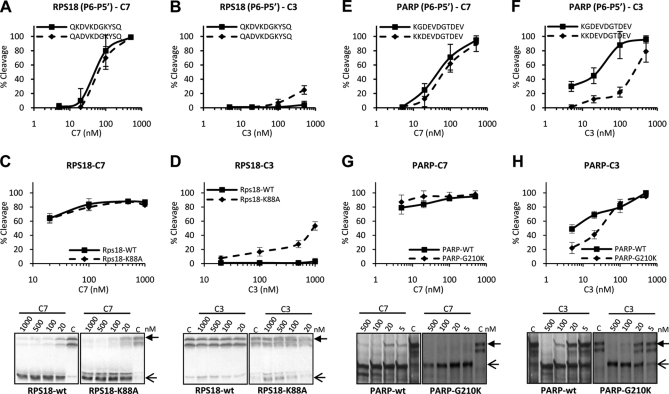
Lysine at P5 position is involved in distinguishing caspase-7 and -3 cleavage. RPS18-wt, PARP-wt, and the respective mutants with modified cleavage sites, RPS18-K88A and PARP-G210K, were transcribed and translated in vitro in the presence of [35S]methionine. The translated proteins and their delineated P6–P5′ undecapeptide (RPS18-wt, QKDVKDGKYSQ; RPS18-K/A, QADVKDGKYSQ; PARP-wt, KGDEVDGTDEV; PARP-G/K, KKDEVDGTDEV) were left untreated or treated with the indicated concentrations of C7 or C3 for 1.5 h at 37 °C and analyzed by autoradiography (lower panels in C, D, G, and H) and densitometry or by RP-HPLC. Cleavage efficiencies of the wild type and K/A-substituted P6–P5′ undecapeptides of RPS18 by caspase-7 (A) or -3 (B), cleavage efficiencies of the wild type and K88A-substituted mutant of RPS18 protein by caspase-7 (C) or -3 (D), cleavage efficiencies of the wild type and G/K-substituted P6–P5′ undecapeptides of PARP by caspase-7 (E) or -3 (F), and cleavage efficiencies of the wild type and G210K mutant PARP proteins by caspase-7 (G) or -3 (H) are shown. A closed arrow indicates the full-length protein. Open arrows indicate the expected caspase-mediated cleavage fragments. Error bars indicate standard deviation of at least three independent experiments.