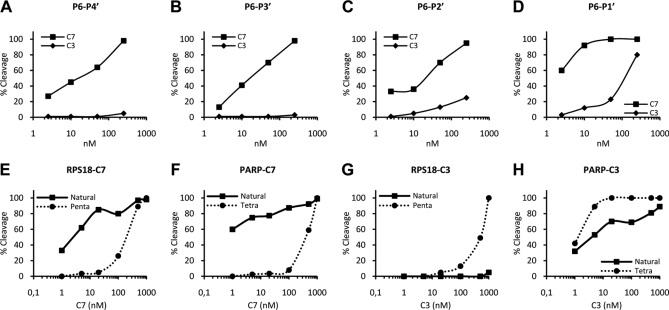
Fig. 6.
Lysine at P2′ and tyrosine at P3′ are involved in distinguishing cleavage by caspase-7 and -3. A–D, the RPS18-delineated truncated peptides (P6–P1′, QKDVKDG; P6–P2′, QKDVKDGK; P6–P3′, QKDVKDGKY; P6–P4′, QKDVKDGKYS) were left untreated or treated with the indicated concentrations of C7 or C3 for 1.5 h at 37 °C and analyzed for cleavage efficiency. Caspase-7 and -3-mediated cleavage efficiencies of the P6–P4′ (A), P6–P3′ (B), P6–P2′ (C), and P6–P1′ (D) peptides are shown. E–H, RPS18 and PARP were transcribed and translated in vitro in the presence of [35S]methionine. The translated proteins and their derived penta- or tetrapeptide caspase substrates were left untreated or treated with the indicated concentrations of caspase-7 or -3 for 1.5 h at 37 °C and analyzed for cleavage efficiency. Caspase-7-mediated cleavage efficiencies of RPS18 and its KDVKD pentapeptide (E) or PARP and its DEVD tetrapeptide (F) and caspase-3-mediated cleavage efficiencies of RPS18 and its KDVKD pentapeptide (G) or PARP and its DEVD tetrapeptide (H) are shown.