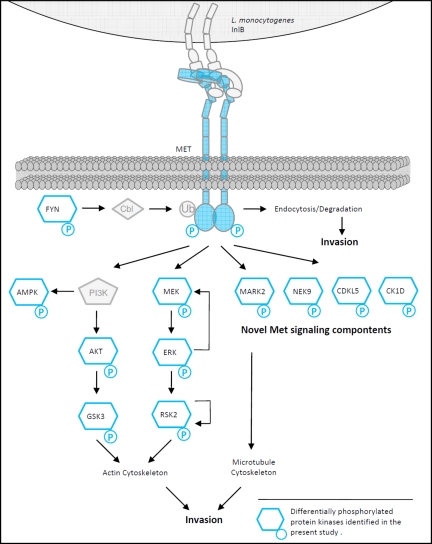
Fig. 7.
Met signal transduction exploited by InlB from L. monocytogenes. InlB321-dependent differentially phosphorylated protein kinases identified in the present study are highlighted in blue. L. monocytogenes activates the PI3K and MAPK pathways, both essential for actin cytoskeleton remodeling as a prerequisite for invasion. The identified induction of phosphorylation events on RSK2 and MEK support a negative feedback theory in this signaling module. Dephosphorylation of MARK2 in the kinase domain as one novel finding is suggested to block MARK2 activation, which is probably essential for Listeria invasion. Active MARK2 leads to destabilization of MTs by phosphorylation of microtubule-associated proteins. Earlier studies already demonstrated that destabilization of MTs by nocodazole impaired efficient Listeria uptake by the host cell. The functional contribution of novel candidates such as Nek9 or CK1D to the InlB/Met invasion strategy will be the subject of further studies. Ub, ubiquitin.