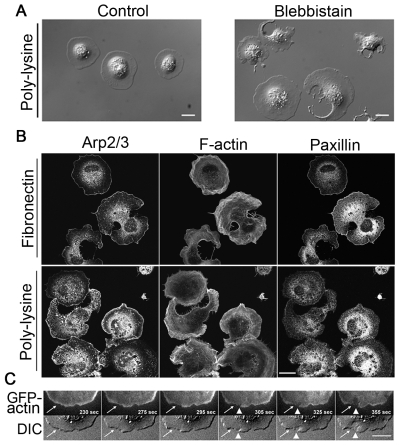
Fig. 5.
Unbalanced actin assembly, not the adhesion of cell to substrates, accounts for cell fragmentation when NMII is inhibited. (A) DIC images of fibroblasts spread on poly-lysine-coated glass for ~20 minutes. Control cells have coherent cytoplasm, whereas blebbistatin-treated cells show loss of cytoplasmic coherence similar to cells spread on fibronectin substrate. (B) Staining for Arp2/3, F-actin and paxillin in blebbistatin-treated fibroblasts spread on fibronectin and poly-lysine substrates. The distribution patterns of Arp2/3 and F-actin are similar in NMII-inhibited cells spread on both substrates. There is little or no accumulation of Arp2/3 along the edge of fragmented sites but there is clear accumulation of Arp2/3 along cell edge of other regions of fragmented cells and along the entire cell edge of unfragmented cells. The actin filaments are wavy in NMII-inhibited cells. Much less F-actin is seen at fragmented regions compared to unfragmented regions. Paxillin does not accumulate along the edge of fragmented regions but does along the edge of unfragmented regions when cells spread on fibronectin. By contrast, paxillin is more diffuse in NMII-inhibited cells spreading on poly-lysine. (C) Top panels are time-lapse images of TIRF GFP-actin and bottom panels are time-lapse DIC images of a cell spreading on fibronectin substrate. White arrows point to the region where the decrease in GFP-actin intensity precedes the initiation of cytoplasmic fragmentation. Arrowheads show the occurrence of cytoplasmic fragmentation. Scale bars: 20 μm.