Abstract
We investigated the relationship between vascular endothelial growth factor (VEGF) expression and clinical outcome in squamous cell carcinoma of the cervix treated with radiotherapy alone. The immunohistochemical study was performed for forty-two paraffin embedded specimens with anti-VEGF mouse monoclonal antibody. Staining was defined as positive for VEGF when more than 10% of the tumor cells were stained from 500 cells counted. Positive VEGF expression was observed in twenty-one among forty-two patients. VEGF expression according to stage (p=0.101), lymph node status (p=0.621), parametrial invasion (p=0.268), and age (p=0.5) revealed no significant difference. But the VEGF expression was significantly higher in tumors larger than 4 cm (p=0.031). Five year survival rates according to VEGF expression status were 89% for VEGF negative group and 47% for VEGF positive group (p=0.02). FIGO stage (p=0.007), tumor size (p=0.025) and the duration of external beam radiation therapy (p=0.006) were also significant prognostic factors for overall survival. We suggest that VEGF expression may be a prognotic factor of the cervix cancer patients treated with radiation therapy alone.
Keywords: Vascular Endothelial Growth Factor, Cervix Neoplasms, Radiotherapy, Prognosis
INTRODUCTION
Radiation therapy plays a critical role in the treatment of cervix cancer (1). Most cases of locally advanced stage disease and patients with poor general conditions receive radiation therapy with curative intent. The response to radiation in the cervix cancer is affected by several factors, and among them, hypoxia is one of the key determinant (2). Hypoxia is known to stimulate vascular endothelial growth factor (VEGF) in cervix cancer cells (3) which stimulates tumor angiogenesis (4) and enhances radio-resistance by targeting vascular endothelial cells (5). Also the induction of VEGF by ionizing radiation is proposed to favor tumor survival by increasing the radioresistance of tumor vascular endothelium (6). Thus the VEGF expression is suggested to have negative influence on patient's survival treated with radiation therapy. There are several reports supporting the prognostic role of VEGF expression in cervix cancer (7-9). These reports suggest possible relationship between VEGF expression and radiation response of cervix cancer. To investigate the relationship between VEGF expression and radiotherapy outcome in cervix cancer, we analyzed forty-two squamous cell cervix cancer patients treated with radiation therapy alone.
MATERIALS AND METHODS
Patient characteristics
The study involved 42 squamous cell cervix cancer patients with available paraffin blocks among the 82 patients who were treated with curative radiation therapy over the period from 1994 to 2001 in Eulji University Hospital in Korea. The histopathologic features of the surgical specimens were classified according to the WHO criteria and the patients were staged according to International Federation of Obstetrics and Gynecology. The staging evaluation included physical examination, tissue diagnosis, cystoscopy, sigmoidoscopy, chest radiography, MRI of the abdomen and pelvis and laboratory studies. The follow-up information was based on the last clinical examination.
The number of patients of stage IB, II and III&IV were 13, 23 and 6, respectively. Median age of the patients was 62 yr (range 28-83). 23 patients had tumor size 4 cm or more and 19 patients had less than 4 cm. Patients with pelvic lymph node on CT or MRI were 20 and patients with parametrial invasion were 23. Median follow-up duration was 39 months (range 3-88).
All patients received at least 45 Gy of radiation therapy in pelvis (range 45-63). Radiation was delivered for five days a week with daily fractions of 1.8 to 2 Gy by 6MV photon. Median duration of external radiation therapy was 43 days (range 30-71). Low dose rate brachytherapy was performed in 28 patients, and median dose to point A was 83 Gy (range 64-104.9).
Immunohistochemical staining
Immunohistochemical staining was performed on archival biopsy material in paraffin block. The microscopic slides were obtained from each block by cutting 5 µm sections with a microtome. The anti-VEGF mouse monoclonal antibody (Oncogene, Boston, MA, U.S.A. diluted 1:10) was used as primary antibody. Antibody binding was detected with a labelled streptavidin biotin (LSAB) kit (ScyTek, Logan, UT, U.S.A.). Antibody binding was done at room temperature for 30 min. After washing, the sections were incubated at room temperature for 30 min with a peroxidase labelled polymer conjugated to goat-anti-rabbit immunoglobulins in Tris-HCl buffer. A substrate-chromogen solution containing 3-amino-9-ethylcarbazole was applied for 5 min. After rinsing in water, the slides were lightly counter-stained with hematoxylin and eosin, dehydrated and mounted. Two pathologists blinded to the treatment of the patients and the outcome interpreted the slides with light microscope. The results were reported as the percent of tumor cells with positive nuclear staining, regardless of the intensity of the stain. Staining was defined as positive when more than 10% of the tumor cells were stained from 500 cells counted. We used placental tissue as positive control and same reagents except the primary antibody for negative control. All slides were evaluated for immunohistochemical staining without knowledge of the clinical outcome.
Statistical methods
A statistical analysis was performed by using SPSS 10.0 for Windows. Survival was analyzed by Kaplan Meier method and compared by log-rank test for univariate analysis. For multivariate analysis, bivariate log-rank test and Cox regression analysis were performed. Survival duration was counted from the first day of radiation therapy to the last day of the follow-up or death. To compare clinical factors and staining results, the chi-square test was used. Probability value <0.05 were considered statistically significant.
RESULTS
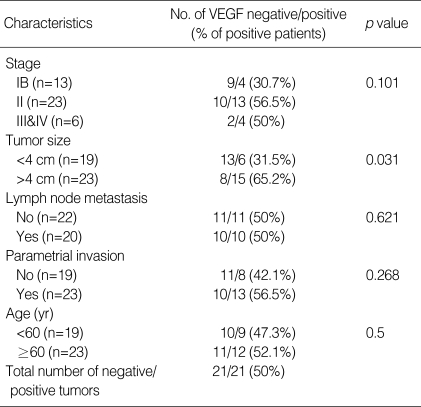
Positive VEGF expression was observed in 21 (50%) of the total of 42 patients. Statistical evaluation of VEGF expression according to stage, lymph node status, parametrial invasion, and age revealed no significant difference. But tumors with 4 cm or more showed significantly high VEGF positive rate (71%) in comparison with tumors less than 4 cm (38%) (p=0.031). All the four patients who died of metastatic disease were VEGF positive. On the contrary, among the nine patients who died with local progression, only one patient was positive for VEGF expression (p=0.008). Correlations between VEGF expression and various clinical features are listed in Table 1.
Table 1.
The relationship between VEGF expression status and other clinical parameters
Five year survival rate for stage IB, II and III & IV were 89%, 67%, and 22% respectively (p=0.007) (Fig. 1). Five year survival rate of the patients with tumor size less than 4 cm was 83% and patients with tumor size more than 4 cm was 53% (p=0.025) (Fig. 2). Five year survival rate according to VEGF expression status were 89% for VEGF negative group and 47% for VEGF positive group (p=0.02) (Fig. 3). Five year survival rate of the patients without detectable lymph node in pelvic CT or MRI was 68% and the patients with positive lymph node was 67% (p=0.8). Among the 13 patients who died, 4 were with metastatic disease and 9 were with local recurrence. The results of univariate log-rank analysis of the relationship between clinical findings and survival are listed in Table 2.
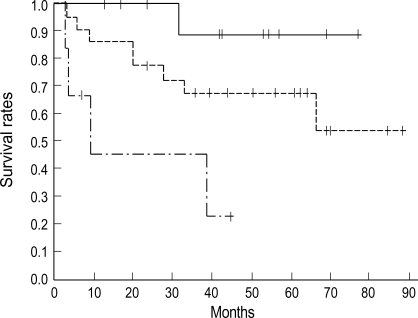
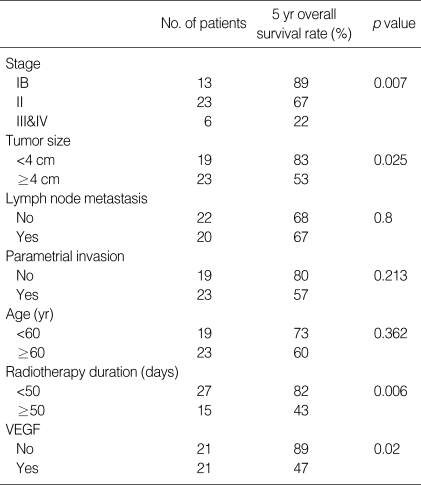
Fig. 1.
Survival curves according to stage. Five year overall survival rate is 89% for stage IB (solid line, n=13), 67% for stage II (dot line, n=23) and 22% for stage III&IV (dashed line, n=6) (p=0.007).
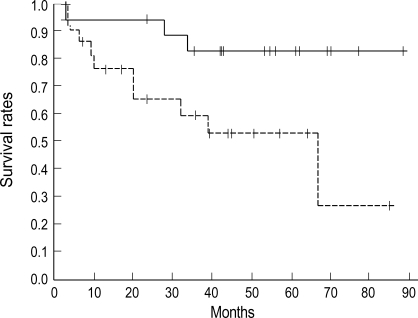
Fig. 2.
Survival curves according to tumor size. Five year survival rate is 83% for the tumors of less than 4 cm (solid line, n=19) and 53% for the tumors 4 cm or more (dot line, n=23) (p=0.0025).
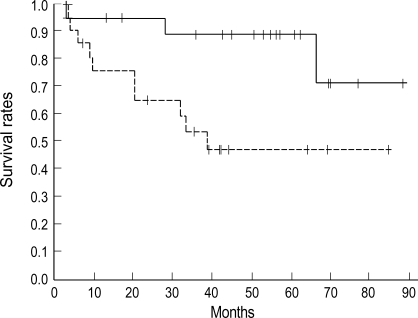
Fig. 3.
Survival of the patients grouped according to VEGF: positive (dot line, n=21) and negative (solid line, n=21). Five year overall survival rate of VEGF negative group was 89% and 47% for positive group (p=0.02).
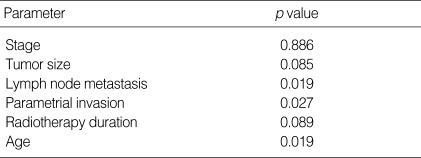
Table 2.
Univariate log-rank analysis of prognostic factors for carcinoma of the cervix treated with radiation therapy alone
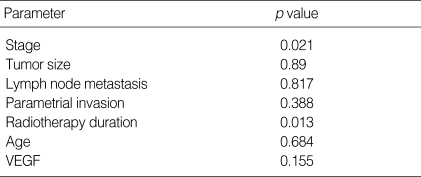
Bivariate log-rank test were carried out in order to test the independence of VEGF expression from other prognostic factors. The significance of VEGF expression remained after stratifying other prognostic factors such as lymph node metastasis, parametrial invasion and age in bivariate log-rank analysis. After stratifying significant prognostic factors in univariate analysis such as stage, tumor size, and radiation therapy duration, bivariate log-rank test did not show statistical significance (Table 3). Cox multiple regression analysis was performed to evaluate the significance of prognostic factors (Table 4). FIGO stage (p=0.021) and radiation therapy duration (p=0.013) were independent prognostic factors for overall survival but tumor size and VEGF expression were not statistically significant in multiple regression analysis.
Table 3.
Bivariate stratified log-rank analyses for the relationship between VEGF expression and clinicopathologic factors after stratifying listed parameters
Table 4.
Cox multiple regression analysis of prognostic factors for cervical carcinoma
DISCUSSION
There are several reports supporting the prognostic role of VEGF expression in cervix cancer but the number of studies about VEGF expression and primary radiotherapy outcome is limited. Loncaster et al. (8) reported that the survival rate was markedly decreased and the distant metastasis rate was increased significantly for the patients with high VEGF expression. According to this report, the VEGF expression was the most potent prognostic factor in multivariate analysis. But the limitation of this study is that they included only bulky stage IB and advanced stage patients to evaluate the relationship between VEGF expression and disease stage, tumor differentiation, patient age and tumor radiosensitivity (SF2) but not tumor size. Cheng et al. (9) reported that the median VEGF concentrations in cervical carcinoma was 118 pg/mL for tumor size less than 4 cm and 1,030 pg/mL for the larger tumors which shows about 9 fold difference between them. It has been reported that serum VEGF was also higher in larger tumors (10). So even in the same stage, it is certain that the tumor size affects VEGF concentration and expression in immunohistochemical study.
There are some debates about the relationship between tumor size and VEGF expression status. Lee et al. (7) reported that the VEGF expression did not have relationship with tumor size. But in this study, all of ten adenocarcinoma patients among total 118 patients belonged to the VEGF low expression group. But VEGF expression frequency and intensity is usually higher in adeocarcinoma than in squamous cell carcinoma of the cervix (11-13). Thus the results of Lee et al. (7) showed unusual expression pattern between adenocarcinoma and squamous cell carcinoma so that we question about the possibility of the unusual ten adenocarcinoma patients disturbed the VEGF expression results in this study. In our study, VEGF expression was significantly higher in tumors larger than 4 cm than in smaller tumors (65% vs. 31%, p=0.031) and also five-year overall survival rate was significantly higher in VEGF negative group (89% vs. 47%, p=0.002). We suggest that the VEGF expression is associated with larger tumor size.
The association between VEGF expression and other clinical findings is controversial. In our study, VEGF expression did not show relationship with clinical factors such as stage, age, lymph node status and parametrial invasion. These results are similar with Loncaster et al. (8), in which there was no correlation between VEGF expression and disease stage, patient age. They did not analyze the relationship between VEGF and lymph node status and parametrial invasion. But Cheng et al. (9) reported that tumors with overexpressed VEGF had higher incidence of deep stromal invasion, parametrial invasion and lymph node metastasis. Our study had limitations that the clinical findings were dependent only on the pelvic examination and pelvic MRI findings because all of the patient received primary radiotherapy and did not receive surgical operations to confirm pathologic findings. We need further investigation about this aspect.
The relationship between VEGF status and disease progression in the previous reports was not in accordance. Bachtiary et al. (10) reported that in patients undergoing primary radiotherapy for cervical cancer, all four patients with local failure had VEGF levels >244 pg/mL, whereas 11 of 19 patients with complete response had serum VEGF levels <244 pg/mL. They suggested that high serum VEGF concentration is associated with impaired response to radiotherapy as well as shortened progression-free survival. Meanwhile, Longcaster et al. (8) reported that high VEGF expression was associated with overall survival and metastasis-free survival, but not local control. They suggested that VEGF expression is primarily reflecting the metastatic potential of a tumor rather than response to radiotherapy. The suggestion that VEGF expression is associated with metastatic potential is supported by Ferrara (14) who demonstrated that VEGF promotes metastatic spread by increasing blood vessel permeability. We did not compare survivals according to the cause of death because of the small number of metastatic death (n=4). However, all of the four patients who died with metastatic disease were positive for VEGF expression. On the contrary, among the nine patients who died with local progression, only one patient was positive for VEGF expression. Our results support the possibility that the VEGF expression is related with metastatic potential rather than local progression.
Our study showed that the positive VEGF expression was significantly related with poor prognostic factors in univariate analysis. The significance of VEGF on the prognosis remained even with the bivariate analysis stratifying clinical factors such as lymph node metastasis, parametrial invasion and age. But in bivariate analysis stratifying previous prognostic factors such as stage, tumor size and radiation therapy duration, the VEGF expression did not show statistical significance. The number of stage III&IV patients (n=6) was too small to be stratified so that the p value (p=0.886) was far from statistical significance. Though the results of bivariate analysis stratifying the tumor size (p=0.085) and the radiation therapy duration (p=0.089) did not reach statistical significance, we suggest further analysis is needed because tumor size was significantly related with VEGF expression in our study. In Cox multivariate analysis only FIGO stage and radiation therapy duration were significant factors for the survival. We suggest that to evaluate whether the VEGF expression is an independent prognostic factor, further investigation with large number of patient is warranted.
References
- 1.Huh SJ, Kim BK, Lim DH, Shin SS, Lee JE, Kang MK, Ahn YC. Treatment results of radical radiotherapy in uterine cervix cancer. J Korean Soc Ther Radiol Oncol. 2002;20:237–245. [Google Scholar]
- 2.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 3.Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 5.Gupta VK, Jaskowiak NT, Beckett MA, Mauceri HJ, Grunstein J, Johnson RS, Calvin DA, Nodzenski E, Pejovic M, Kufe DW, Posner MC, Weichselbaum RR. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 2002;8:47–54. doi: 10.1097/00130404-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 7.Lee IJ, Park KR, Lee KK, Song JS, Lee KG, Lee JY, Cha DS, Choi HI, Kim DH, Deung YK. Prognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2002;54:768–779. doi: 10.1016/s0360-3016(02)02970-x. [DOI] [PubMed] [Google Scholar]
- 8.Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000;83:620–625. doi: 10.1054/bjoc.2000.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng WF, Chen CA, Lee CN, Chen TM, Hsieh FJ, Hsieh CY. Vascular endothelial growth factor in cervical carcinoma. Obstet Gynecol. 1999;93(5 Pt 1):761–765. doi: 10.1016/s0029-7844(98)00505-5. [DOI] [PubMed] [Google Scholar]
- 10.Bachtiary B, Selzer E, Knocke TH, Potter R, Obermair A. Serum VEGF levels in patients undergoing primary radiotherapy for cervical cancer: impact on progression-free survival. Cancer Lett. 2002;179:197–203. doi: 10.1016/s0304-3835(01)00872-2. [DOI] [PubMed] [Google Scholar]
- 11.Santin AD, Hermonat PL, Ravaggi A, Pecorelli S, Cannon MJ, Parham GP. Secretion of vascular endothelial growth factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix. Obstet Gynecol. 1999;94:78–82. doi: 10.1016/s0029-7844(99)00282-3. [DOI] [PubMed] [Google Scholar]
- 12.Tokumo K, Kodama J, Seki N, Nakanishi Y, Miyagi Y, Kamimura S, Yoshinouchi M, Okuda H, Kudo T. Different angiogenic pathways in human cervical cancers. Gynecol Oncol. 1998;68:38–44. doi: 10.1006/gyno.1997.4876. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Kim HS, Jung JJ, Lee MC, Park CS. Expression of vascular endothelial growth factor in adenocarcinomas of the uterine cervix and its relation to angiogenesis and p53 and c-erbB-2 protein expression. Gynecol Oncol. 2002;85:469–475. doi: 10.1006/gyno.2002.6648. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]